Genetics & Periodontal Disease
The good news is that the list of acknowledged and proven periodontal risk factors is relatively short and well-understood.
Thomas M. Hassell, DDS, PhD, and
Trisha O`Hehir, RDH, BS
Viewed globally, the oral-health care professions face only two adversaries: dental caries and periodontal disease. On the one hand, the hereditary basis for susceptibility to caries is rather well-founded, and mechanisms for long-term caries prevention, including systemic and topical fluorides, have been generally acknowledged for decades.
On the other hand, the genetics of susceptibility to inflammatory periodontal diseases have remained elusive. This fact derives from the complexity of the disease, the continuous emergence of new knowledge about its pathogenesis, the relative contributions of a myriad of microorganisms to its etiology, and the vagueness of clinical diagnosis and statistical quantification. It is also a result of the periodontal community`s own penchant for arcane and ever-changing nomenclature to classify the periodontal diseases, which continues to evolve today.
The theory that host genetic make-up may act in combination with environmental factors to influence periodontal disease is not new. In 1930, it was concluded that susceptibility and immunity to periodontal disease were "probably inherited." Even earlier, the German-language dental literature provided a series of undocumented, but nonetheless intriguing, quotations on the topic:
"Pedigree analyses reveal again and again that blood relatives often suffer from pyorrhea." (Moral, 1924)
"I often have observed that the parents of children with advanced pyorrhea lost their teeth early." (Sachs, 1925)
"The susceptibility to true alveolar pyorrhea is due to a specific lack of host resistance, which is most likely not acquired, but inherited." (Reinmoller, 1925)
"Inheritance plays a massive role in periodontitis. I once saw a mother of eight who had periodontal disease, and so did four of her children." (Boenheim, 1928)
In the face of a known pathogenic challenge, some people succumb, while others seem inherently resistant. It has been recognized since the earliest days of dentistry and medicine that some diseases or conditions are familial or "run in families." As an example of this phenomenon, let`s consider tobacco smoke and lung cancer.
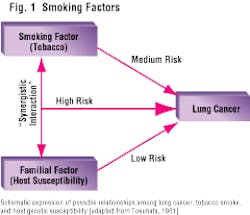
Fact: Not all people who smoke develop cancer. And yet, tobacco smoke contains over 4,700 chemical compounds, over 40 of which are acknowledged carcinogens. Thirty years ago, Dr. Tokuhata studied lung cancer in several thousand smokers, nonsmokers, and their relatives. He provided an instructive and revealing diagram (Figure 1), which implicated a "familial factor" in the pathogenesis of lung cancer. His diagram shows the synergistic interaction between the familial factor and the tobacco factor. People who have both of these factors are subjected to a dramatic increase in lung cancer. Dr. Tokuhata referred to this as a "booster factor."
Ten years later, other scientists were able to explain this phenomenon when they discovered that some people have a genetic ability to convert potential (tobacco-derived) carcinogens into active carcinogens, while other people do not. Members of the latter group are less susceptible to the disease, even though they are exposed to the same carcinogens - tobacco by-products.
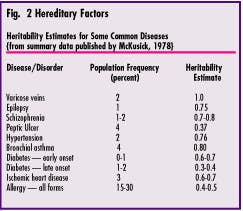
Subsequent research - much of it performed by Dr. Victor McKusick - led to the assignment of what is now known as the "heritability estimate" for many medical conditions. This is a quantitative measure of the extent to which a person`s genetic constitution can be held "responsible" for the fact that disease develops when the challenge is present. Figure 2 provides the Heritability Estimates for some common diseases. It reveals that varicose veins, for example, are 100 percent heritable (not at all influenced by environmental factors), while stomach ulcers are only about 35 percent heritable. Recent clinical research has demonstrated that the Heritability Estimate for clinical signs of periodontal disease probably ranges between 40 and 80 percent. That means that when challenged by the presence of pathogen-containing dental plaque, some people will develop periodontitis, while others will be resistant to it.
Human twin research
In post-war Bonn, Germany, periodontal disease was studied by means of the "human twin paradigm," which had been employed for clinical research in medicine and psychology since 1876. In 13 sets of identical twins over the age of 25, severe periodontitis was detected in four twin sets. Both twins in the four twin sets were affected and concordant for relevant clinical parameters including pocket formation, gingival recession, subgingival calculus accumulation, tooth mobility, and periodontal pocket suppuration. The researcher was quick to acknowledge that his sample size was quite small, but he suggested that his preliminary findings should stimulate expanded studies of adult twins in periodontology, and should include non-identical (fraternal) twins as the ideal control group.
Almost 40 years went by before this advice was heeded by the community of periodontal scientists. Thus, only since 1989, and up until the present time, have clinical examinations of human twins contributed new knowledge about the major role that genetics plays in periodontal disorders.
Today, twin studies typically are used to detect genetic variance for traits or conditions that are multifactorial. The comparison of the similarity of identical twins with that of fraternal twins is based on the difference in shared genes and the similarity of shared environments, so that a greater degree of similarity for identical twins is evidence for genetic variance. For the simple model on which estimates of heritability are based for Mendelian traits, you would expect the correlation for identical twins to be no more than twice that for fraternal twins. However, this does not always hold true for multigenic traits. An even greater difference between the similarity of identical and fraternal twins suggests that a more complex genetic model - gene-environment interaction - or greater shared environment for identical twins is a better explanation of the observed correlations. A near-equal correlation for identical and fraternal twins suggests that shared environmental factors - not genes - account for any observed similarities.
Since 1984, dental researchers in Minnesota also have studied twins reared apart. Presumably, such twins have no more shared environment than any two unrelated individuals, and certainly share less of their environment than do twins reared together. Thus, including twins reared apart in a study design facilitates stronger conclusions about the importance of genetic variation and shared environment in familial clustering in a disease process.
Thirty years ago, a clinical study of periodontal health in 26 twin pairs aged 12-17 years found no evidence of a genetic contribution to variation in gingival recession, sulcus probing depth, or indices of gingivitis, calculus, and plaque. However, more recent and well-controlled studies of more appropriately-aged twins support the concept that genetic variation may contribute to individual differences in risk for the more common adult periodontitis.
For example, in a large, population-based twin study of questionnaire-assessed periodontal disease, identical twins were found to be more concordant for periodontal disease than were fraternal twins. Thus, if one is an identical (monozygous) twin, the risk of developing periodontal disease if one`s sibling has had periodontal disease is 28-31 percent vs. 8-16 percent for fraternal twins. Both of these concordance rates are higher than the prevalence of periodontal disease among twins (5 percent) and among non-twin spouses (4 percent). However, age and gender differences in the risk for periodontal disease - and other risk factors for periodontal disease (e.g., smoking) - were not specifically taken into account in these analyses.
Twin similarity for clinical measures of periodontal disease and for potential host-risk factors for periodontal disease has recently been assessed. After age and gender effects on each measure were taken into account, the similarities of identical twins reared together, fraternal twins reared together, and identical twins reared apart were compared for attachment loss, pocket-probing depth, gingival index, and plaque index. The greater similarity of identical twins - whether reared together or apart - suggests that there is a genetic contribution to variations in levels of supragingival plaque and clinical measures of periodontal health. However, the low degree of similarity in fraternal twins for most measures suggested that the genetic model may not be a straightforward additive model, but may instead be an interaction between genes at one locus (dominance), among genes at more than one locus (epistasis), or between genes and other risk factors.
The difference in similarity of alveolar bone height for identical twins reared together and fraternal twins suggests that genetic variation contributes to individual differences in alveolar bone height. The difference in similarity between identical twins reared apart and those reared together suggests that shared environmental factors also contribute to variation in alveolar bone height.
In-vitro biology
Another field of science that has emerged relatively recently is the field of in-vitro biology. In-vitro biology has permitted periodontal scientists to ask relevant questions about the possible genetic mechanisms operating to control periodontal disease susceptibility. The ability to harvest human cells - blood cells and somatic cells - and to maintain their viability in isolation in the laboratory under various experimental conditions has provided a fertile landscape for experimentation and hypothesis-testing.
For example, scientists studied the ability of human-blood neutrophils in vitro to migrate through a filtering membrane toward a known chemotactic substance. As a result of these studies, they were first able to ascertain that a cell-migration defect - i.e., inability or slowness of movement - represented one component of the pathogenesis of a form of periodontal disease. This form is known to have a genetic base. It is termed juvenile periodontitis, namely the inadequate host response to etiologic substances.
In-vitro studies of human gingival and periodontal connective tissue cells - known as fibroblasts - have contributed much to our understanding of tissue destruction and regeneration. In-vitro investigations of alveolar bone cells - called osteoblasts - provided breakthrough information about the roles played by various byproducts of microorganisms in the initiation of bone resorption.
The in-vitro method permits exposure of cells from identical and fraternal twins to various exogenous agents to assess whether response to such agents could be under genetic control. The first exploration of this hypothesis evaluated the response of gingival fibroblasts from twins to the anti-epileptic drug, phenytoin (Dilantin). It showed that the effect of phenytoin on general-protein synthesis - and specifically on collagen synthesis - was under strong genetic control. The researchers concluded that the gingival enlargement and overgrowth often elicited by phenytoin also is a genetically determined response. Clearly, not all people who take phenytoin on a chronic regimen develop gingival overgrowth; only those who are genetically susceptible do so.
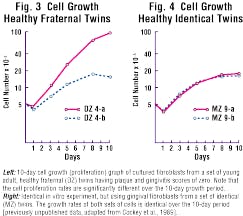
Both homeostasis and repair of periodontal soft tissues are regulated for the most part by resident fibroblastic cells. An important attribute of such cells is their ability to proliferate when necessary to replace lost or damaged tissues. In-vitro study of fibroblasts derived from twins has demonstrated that the cellular-proliferation rate of these cells is under strong genetic control (see Figures 3 and 4).
The concept of a cellular basis for genetic susceptibility to adult periodontitis also has been subjected to laboratory investigation by means of the in-vitro twin paradigm. Since 1960, numerous authors have studied the effects of putative periodontal-pathogenic microorganisms and their byproducts upon human cells in culture. Over 30 different microorganisms have been identified as putatively pathogenic; thus, some researchers have tried to ascertain the effects of such microorganisms upon cultured cells derived from human oral tissues. One advantage of the in-vitro method is that individual microorganisms can be studied in isolation. For example, Treponema denticola was shown to attach to cultured epithelial cells, while Porphyromonas gingivalis had deleterious effects on the proliferation rate of human gingival fibroblasts. The byproducts of Porphyromonas gingivalis also altered fibroblast morphology.
Based on these findings, it follows that susceptibility of gingival connective tissue cells to the effects of oral microorganisms is a genetically influenced trait. To test this hypothesis, paired twins` gingival fibroblasts were exposed to bacterial extracts. Control cultures revealed cells with intact connective tissue fibers perfectly lined up and appearing to actively migrate within the culture dish. However, after exposure to bacterial extracts, the more diffuse or clumped the cells became. Extended exposure resulted in frank disruption of the cell membranes of cultured fibroblasts. The similarity between cells of identical twins was greater than that for cells of fraternal twins. This finding is consistent with a significant genetic influence on individual variation of response to bacterial challenge. With statistical analysis, identical twins showed significant similarity while cell cultures from fraternal twins exhibited no similarity.
It is now clear that the susceptibility to alveolar bone loss is not under simple Mendelian control. The mechanisms are multigenic and complex, with environmental factors (pathogenic microorganisms, smoking, stress etc.) playing a significant role in whether the trait "alveolar bone loss" is expressed or not. Genetic control of periodontal disease resistance or susceptibility could be exerted through many different biologic pathways.
Another genetic study of human twins revealed significant genetic control of salivary proteins (e.g., lactoferrin, lysozyme, peroxidases, and secretory IgA) that have important antimicrobial properties. These components of human saliva can significantly influence the colonization and growth of periodontal pathogenic microorganisms. Thus, a genetic defect resulting in subnormal salivary levels of such antimicrobial substances could render the host more susceptible to bacterial colonization and subsequent periodontal breakdown.
The twin model for studying periodontal disease susceptibility has been used very little thus far. It is a powerful technique - both in vivo and in vitro - for dissecting out hereditary factors in disease pathogenesis, and future research efforts should be targeted toward exploiting this well-established methodology.
Clinical predictive testing
Although large clinical studies of family groups and twins have provided valuable evidence of a strong hereditary component of periodontal disease susceptibility, such studies do not provide information about individual susceptibility. The inability of clinicians to determine the future course of a patient`s periodontal health leads to treatment decisions that are based more on intuition than on scientific fact. Inevitably, many patients are either undertreated based on the lack of objective findings at the time of clinical examination or they are overtreated in an attempt to make sure the best possible level of periodontal health is achieved.
Several "high tech" methods for periodontal diagnosis have appeared on the market over the past decade, such as tests for the presence of putative pathogenic microorganisms, tests for enzymes, host-derived tissue-breakdown products, and inflammatory mediators. Such tests are, indeed, applicable to individual patients. However, these tests are almost exclusively diagnostic, not predictive. That is to say, clinical and biological evaluations can tell the dentist and dental hygienist about the current status of the patient`s periodontium, but these signs, symptoms, and clinical judgments have very weak prognostic value.
For example, it is a relatively straightforward clinical process to diagnose moderate and advanced periodontitis. However, up until very recently, no mechanism existed for determining which patients with mild or no disease will respond negatively to bacterial plaque and then progress to a more severe periodontitis that demands more extensive clinical treatment. The lack of reliable markers for patient susceptibility to severe periodontitis has, until now, prevented early identification of those persons who are most at risk, and it has also prevented the delivery of therapy that would be appropriate in view of the individual risk.
To date, clinical treatment for periodontitis has been based upon the so-called "Plaque-Antiplaque Model," which is a simple, three-point hypothesis:
(1) Periodontitis treatment is antibacterial because bacteria cause the disease.
(2) Specific and nonspecific bacteria are identified as the antibacterial targets.
(3) Reduction, control, and elimination of sites conducive to bacterial colonization are effective treatments for most patients.
Over the years, this model was given considerable support from the scientific community, and it has been the driving force for many clinical treatment decisions. The major difficulty with the plaque-antiplaque model is its failure to take into account the fact that, while specific bacteria are necessary for disease development, bacteria alone are not sufficient to cause disease. In other words, the presence of bacteria does not automatically mean that the patient will have the disease.
This reminds us of the 1964 paradigm, established by Dr. Tokuhata, for smoking and lung cancer: Tobacco alone does not cause the disease; rather, there is a synergistic interaction between tobacco and one or more familial-host susceptibility factors. The same paradigm can be applied to periodontal diseases. (See Figure 5.)
The bacterial etiology of periodontitis, while important, cannot explain all of the variability we see in practice. Some patients have abundant plaque and little or no disease; other patients have only a small amount of plaque, but suffer severe disease. Many patients respond well to standard, conservative periodontal therapy, but some patients do not. Therefore, universal application of a standard-treatment method - antiplaque therapy - to every patient results in a mix of good and not-so-good clinical results.
A recent advance in periodontal research has provided oral health care practitioners with a new and powerful tool for early detection of disease susceptibility. By means of a simple blood test, it is now possible to ascertain early on whether a patient - who might present in the dental office today with healthy-appearing periodontal tissues - is genetically destined for periodontal destruction. The finger-stick test, and more recently, a saliva test, represent the most contemporary technology of research at the level of the gene, permitting genetic analysis of how an individual can be expected, in the future, to respond to challenges by known periodontal, pathogenic microorganisms and their byproducts. The test is called "PST," for Periodontitis Susceptibility Test, and is available from Interluken Genetics. The test does not diagnose currently active disease; rather, it is an assessment of whether a patient is likely to develop severe periodontitis in the future.
The test actually analyzes specific genetic markers associated with increased Interleukin-1 (IL-1) production. This suggests a genetic mechanism by which some individuals, if challenged by bacterial accumulations, may have a more vigorous immuno-inflammatory response that leads to more severe periodontitis. These "markers" for disease actually are functional. Monocytes from patients who are genotype-positive produce more IL-1 when stimulated by bacterial antigen. This is significant because the pathologic process resulting from increased IL-1 has been strongly associated with the pathology of periodontitis. Production of IL-1 is believed to lead to more generalized and severe disease.
Despite this genetic risk factor, smoking far outweighs the IL-1 genotype as a pathogenic consideration; that is, smoking is a more significant risk factor than being IL-1 genotype-positive. For patients with greater than a 20 pack-year history of smoking, the impact of the smoking itself is such a dominant environmental factor that it eclipses much of the genetic influence in determining disease severity. It is likely that smoking, combined with other risk factors, can have some additive effects, but scientific evidence for this has not yet been published.
Additionally, most of the IL-1 genotype research to date has looked at people of Caucasian Northern-European heritage. Thus, the direct applicability of these data to other racial and ethnic groups is subject to question.
Conclusions
The list of acknowledged and proven periodontal risk factors is relatively short and well-understood: smoking, stress, compromised immune system, and poor oral hygiene.
Clinical and laboratory research over the past decade, including family-group studies, the human-twin paradigm, and in-vitro evaluation of human cellular responses to bacteria, have confirmed that there is a strong genetic component of susceptibility to periodontitis and periodontal destruction. This permits adding a fifth Orisk factorO to the list: genetic susceptibility due to the IL-1 genotype.
Three of these five major risk factors can be eliminated or modified:
x A patient can elect to stop smoking.
x A patient can improve oral hygiene.
x A patient can take measures to reduce the level of stress (or take stress-reducing medications).
The risk factor of a significantly compromised immune system is, fortunately, quite rare; it seems to become manifest only in those few patients who suffer juvenile or early-onset forms of periodontitis.
The risk factor of genetic susceptibility due to IL-1 genotype is predictive for future severe disease, and it is estimated that 30% of the (Caucasian) population is positive for the IL-1 genotype. This leads to a new paradigm in periodontics, based on risk and susceptibility, rather than on plaque-antiplaque exclusively. This will elicit behavioral changes in both clinicians and the patients they treat.
Traditional concepts of treatment and prevention, which have relatively little prognostic or diagnostic value, will be replaced by more pro-active health and disease management, wherein risk and predisposition assessment will be used as first priority decision-making guides. Clinicians will be able to identify and monitor periodontal risk much earlier, and this will improve the opportunity for successful therapeutic outcomes. This also will maximize cost-to-benefit equations.
Periodontics today finds itself at the leading edge of biomedical science in terms of genetic-risk assessment. The dental and dental hygiene professions are poised to take advantage of new scientific evidence to improve the quality of life for their patients.
Thomas M. Hassell, DDS, Dr. med. dent., PhD, is affiliate professor of periodontics at the University of Washington, Seattle, Wash., and principal scientist of the Optiva Corporation, Snoqualmie, Wash. Trisha O?Hehir, RDH, BS, is a senior consulting editor for RDH magazine and the Editor of Perio Reports.