Break the chain of infection in your dental practice
by Patricia Pine, RDH
Monday mornings are usually crazy days for any oral surgery office. This office was no different. There was a full Monday, which included extractions, implants, and emergencies. The 9:45 a.m. patient, Brenda, was scheduled for three routine extractions. Brenda was concerned about her extractions. She couldn't wait to get these teeth out, as they were decayed below the gum line. The 2:00 p.m. patient, Suzie, was scheduled for four wisdom teeth extractions. Suzie, a lovely 60-year-old patient, was nervous about her treatment. All treatments for the day seemed to progress smoothly. No glitches -- a smooth Monday schedule.
Four months later the office was shocked to see the Centers for Disease Control (CDC), local health department, and OSHA at the door. The dentist was informed that the 60-year-old patient Suzie, who had four teeth extracted four months ago, had developed symptoms of hepatitis B. Suzie had no lifestyle risk factors, which caused the beginning of an investigation with the health department along with the CDC. They were backtracking her lifestyle and discovered her extractions could be a viable source of infection. When investigators visited the office, they monitored everyone's daily operations and every move. Instrument handling, delivery of IV medication and anesthesia cartridges, instrument storage, and sterilization procedures were exhaustively evaluated. They questioned each staff member individually on office protocol. They scrutinized who did what, when, where, and why.
-----------------------------------------------------------
Consider reading:
-----------------------------------------------------------
Concerns of infectious diseases in dentistry involve HIV, HBV, HCV, and the seasonal flu. HIV does not spread or maintain infectiousness outside the host. Hepatitis B virus can live on an environmental surface for up to seven days in dried blood or contaminated instruments. Always place contaminated instruments in the ultrasonic cleaner to prevent debris and bioburden from drying on them. Hepatitis C can live at room temperature for 16 hours or four days, depending on the situation. This virus is spread through contact with infected blood. This can occur through a small cut on the hands, an open nail cuticle, or an open wound. Flu virus survives on a surface for 48 hours. A sneeze travels at a speed of 100 miles per hour. Just think of all the sneezing in the operatory that enables the spreading of a variety of viruses on environmental surfaces.
As dental professionals, we must clean and disinfect all countertops and equipment between patients with a recommended hospital-grade bactericidal product according to the manufacturer's instructions. Nonvisible blood and saliva does not mean it is not present on environmental surfaces. Blood is mixed with saliva throughout dental treatment.
The CDC found the office in question followed standard infection control practices and all staff had been previously vaccinated and tested negative for HBV. The investigators could only speculate that a lapse in cleanup procedures had occurred after the source patient had been treated. The 9:45 a.m. patient, Brenda, had acute hepatitis B (unknown to the office). She was on the state's reportable disease registry for HBV. What went wrong? How did the HBV-infected patient from the morning appointment infect a patient in the afternoon? How could this have been avoided? What techniques can the entire dental community provide to prevent this transmission from occurring in the future? Could this happen in your practice?
Understanding and recognizing the risk of disease will reduce practice liability, patient illness, and employee illnesses. Dentists cannot pretend that this is only a theoretical problem. The dental community needs to stay abreast of the rapidly evolving standard of care and do its best to comply with the standard of care in daily practice. If a patient becomes ill as a result of an exposure to a pathogen or a bacterium that is later found on your dental surfaces, the employer/dentist is at risk of being held responsible for the patient's illness and associated compensatory damages.
Breaking the chain of infection would be an avenue in reducing the spread of infectious disease. Covering one's cough or sneeze with a sleeve -- not the hands -- is the CDC's recommended approach. Hands touch many objects throughout the day and germs are easily transferred to surfaces, causing cross-contamination. Bacteria will die inside the material of clothing. Sneeze into a sleeve, covering both the nose and the mouth. Another way to break the chain of infection is through frequent hand washing. The following four things are necessary for infection to occur:
- The presence of a pathogen (infective agent) in sufficient quantity to cause infection
- A source of infection -- human, animal, insect, environment
- An entry point/mode of transmission -- e.g., an open wound
- A person's (host's) susceptibility to the pathogen
The type of virus or bacterium determines what type of infection is transferred from person to person. The Centers for Disease Control (CDC) requests that all health-care professionals receive a flu vaccine to assist in preventing the transmission of the flu. Many different types of flu have emerged within the last 10 years. Consult your primary care provider for guidance.
Environmental infection control
Certain surfaces, especially ones touched frequently (e.g., light handles, unit switches, and drawer knobs) can serve as reservoirs of microbial contamination, although they have not been associated directly with transmission of infection to either dental health-care professionals (DHCP) or patients. In the story above, hepatitis B transmission did occur in October 2001 after routine extractions. Cleaning and disinfecting environmental surfaces can be a life or death task for patients in any dental facility. Transfer of microorganisms from contaminated environmental surfaces to patients occurs primarily through DHCP hand contact. When surfaces are touched, microbial agents can be transferred to instruments, other environmental surfaces, or to the nose, mouth, or eyes of workers or patients. Although hand hygiene is key to minimizing this transferal, barrier protection or cleaning and disinfecting of environmental surfaces also protects against health-care–associated infections.
Environmental surfaces are divided into clinical contact surfaces and housekeeping surfaces. Housekeeping surfaces such as floors, walls, and sinks have limited risk of disease transmission. Action plan for cleaning and disinfecting surfaces in patient-care areas should consider written standard operating procedures that consider the following:
- The potential for direct patient contact
- The degree and frequency of hand contact
- Potential contamination of the surface with body substances or environmental sources of microorganisms
Cleaning is the necessary first step of any disinfection protocol. Cleaning is a form of decontamination that renders the environmental surface safe by removing organic material and visible soils, all of which interfere with microbial inactivation. If a surface has not been cleaned first, the success of the disinfection process can be compromised. If a surface cannot be cleaned adequately, it should be protected with barriers. Barrier protection of surfaces and equipment and small buttons or switches can prevent contamination of clinical surfaces including those hard to clean.
Let's not forget bib holders. Contaminated bib holders typically are placed with bare hands. The North Carolina Dental School has done intensive studies to provide us with results that none of us wanted to see. All types of bib holders are highly contaminated with strep, staph, dead skin cells, and E. coli. You can review the article here: www.burkhartdental.com/sites/default/files/Pathogens.pdf.
When barriers are not being used, surfaces should be cleaned first, then disinfected between patients using an EPA-registered hospital disinfectant with an HIV, HBV claim (low-level disinfectant) or a tuberculocidal claim (intermediate-level disinfectant). An intermediate-level disinfectant should always be used when a surface is visibly contaminated with blood or other potentially infectious material (OPIM). As stated earlier in this article, environmental surfaces can become contaminated via sneezing and coughing, which can contaminate work surfaces. This, in turn, can cause the transmission of disease. Potentially infected bacteria or blood and saliva cannot always be seen. Treatment areas should be kept free from clutter (e.g., papers, supplies, and equipment) to expedite cleaning and disinfecting treatment areas between patients.
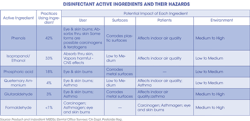
Mycobacterium TB (Mtb) has been a benchmark for dentistry for years. Mtb is a bacterium that is hard to kill because of its cell wall, which is 60% lipid. This TB bacterium is an aerobe; it needs oxygen to survive and can withstand weak disinfectants and survive in a dry state for weeks. Mtb isn't transmitted via contaminated environmental surfaces, so why is it so important for a disinfectant to be tuberculocidal? This is confusing to many. TB is transmitted via an airborne route. Potency against Mtb has been recognized as a substantial benchmark to measure germicidal potency. Mycobacterium has the highest intrinsic levels of resistance among the vegetative bacteria, viruses, and fungi; germicides with a tuberculocidal claim on disinfectant labels are considered capable of inactivating a broad spectrum of pathogens, including such less-resistant organisms as bloodborne pathogens (e.g., hepatitis B virus, hepatitis C virus, and HIV).
Different products have different TB claims. Disinfectant sprays may have a longer kill time than a disinfectant wipe. Be sure to check kill times on each product along with their safety data sheets (SDS).
Perfect disinfectant -- keep in mind that each dental practice has different types of clinical surfaces: contaminated instruments, contaminated bib holders, hands, gloves, protective eyewear/face shields, and masks directly touch these surfaces. Sorry to say, there is no perfect disinfectant. Evaluate your choice of disinfectant prior to purchase; view the MSDS -- now known as safety data sheets (SDS).
What is the current kill time on your disinfectant? Always follow the manufacturers' directions.
High technology has provided dentistry with infection-control–friendly equipment. Cleankeys is a flat keyboard that saves time when cleaning and disinfecting any operatory or for the administrative staff. This style of keyboard eliminates worries about spilled liquids or food lodging in between the keys. This keyboard has a mouse included to guard against contamination and promote easy cleaning.
The Dental R.A.T. is a hands-free, voice-activated periodontal probing system designed by a dental hygienist to enhance care while reducing the risk of contamination. Please view the video on the dentalrat.com website for more information. This technology puts a whole new light on discovering periodontal disease. Discover how quick and easy this technology can assist in a day's work.
What personal protective equipment is necessary when disinfecting?
According to OSHA, when using chemicals employees must wear utility gloves for protection. Some manufacturers state gloves are "not necessary." I suggest safety first. Let's think about this. Since we know that our skin can absorb chemicals, why wouldn't we want to protect ourselves? Why risk your health over a pair of utility gloves?
Utility gloves are washed and dried and placed on a hook for use between each patient. Some utility gloves can be sterilized weekly. Clinical gloves are known to have microscopic holes in them. This is why washing hands before and after using gloves is so important. Utility gloves are used for cleaning and disinfecting contaminated operatories and during all aspects of sterilization. Regular dishwashing utility gloves purchased at the local grocery store are not appropriate. They cannot be sterilized.
- Clinical gowns are recommended per OSHA to protect workers' skin on arms and necks from the splash and splatter of bloodborne pathogens, chemicals, and OPIM.
- For masks, single-use protection is required for any clinical workday during patient care with splash and splatter, while cleaning operatories, and for sterilization of instruments. There is a mask for every task.
- Off-label use includes using disinfectants on 4x4s in any type of container is using this product off-label. It has been determined that using 4x4s soaked in disinfectant solution interacts with the bleached cotton and deactivates the disinfectant. So why bother? If the disinfectant manufacturer states on the instructions that their product can be used in this manner, then by all means, it can be done. Please read the manufacturer's instructions prior to purchase and use. Studies have shown that fibers in the gauze can bind up the active ingredients in the disinfectant, rendering it less effective or ineffective. Instead, a dry wipe can be sprayed or soaked at time of use. Ready-made presoaked wipes are more time efficient and effective. Don't guess; know that your disinfectant is working.
How to use disinfectant wipes
Place utility gloves on bare hands. Pull out the number of wipes needed (3-4+). Pull wipes away from you, to prevention splashing in face or eyes. The type of surface and surface area that needs disinfecting will determine the number of disinfectant wipes needed. Close the lid with the clean wipe. Keep lid closed to prevent moisture loss.
Clean all required surfaces and equipment. Use another clean wipe to disinfect surfaces just cleaned. In the absence of heavy soil, take a clean wipe and thoroughly wet surface for full kill time stated on the product label. Treated surface must remain visibly wet for the full TB kill time. Use additional wipes if needed for continuous contact time. High alcohol disinfectants may evaporate quickly; surfaces must remain visibly wet for entire TB kill time per product instructions. Allow the surface to air dry.
Turning disinfectant container over during lunch or at the end of the day will keep towelettes moist.
Spray-wipe-spray is a technique that is exactly what it states: spray–wipe–spray. The surface must remain visibly wet for the total kill time required. This is very important for disinfectants to be successful.
Could your team be getting headaches from using the disinfectant? Chemicals can do strange and hazardous things to the human body. Many times concern can be about a chemical, yet unknowingly other causes can affect the human body. Fingernail polish is beautiful on fingernails, but are you aware that nail polish can contain formaldehyde, a carcinogen, in the polish?
Please view your current disinfectants' MSDSs. What are the health concerns? How hazardous could any chemical be to the health of your team?
Assist in new team-member training as well as keeping the team on the same page. Written protocols provide easy, step-by-step directions for all staff members to view. This will establish protocols and encourage effective and consistent procedures.
Remember to wipe down phones, keyboards, mouse, and use a special cleaner for monitors. Each facility needs to create a schedule for cleaning. Weekly is good, but daily is great. Team members will stay healthier.
Dentistry has come a long way with surface disinfectants that are safer and easier to use. Each product needs to be used according to manufacturer's directions, reading the entire label for instructions. Print the SDS and review before purchase of any chemical product. Any new product should be reviewed with all aspects of chemical use, especially health hazards, at a monthly team meeting so all team members are on the same page prior to purchase. Listed are several products for your convenience. When dissecting your disinfectant, look for kill times that meet the needs of your practice, health concerns, and learn which pathogens will be eliminated. Check for odors and any hazards. All employees have the right to work in a safe environment. RDH
References
CDC Centers for Disease Control and Prevention, Hepatitis B Virus Transmission in a Dental Office
CDC Guideline for Disinfection and Sterilization in Health Care Facilities, 2008
Surface Disinfectants for the Dental Office
U.S. Environmental Protection Agency -- Office of Pesticide Programs
Organization for Safety & Asepsis Procedures
Crosstex -- General guidelines for use; Language both English and Spanish
Patricia M. Pine, RDH, is a dental hygienist, international speaker, and creates in-office training for dental professionals. She may be reached at [email protected] for consulting and presenting opportunities, or visit her website at www.OSHAtrainingbootcamp.com.
What should you look for in a disinfectant?
- EPA registration number
- Labeled "Hospital Disinfectant"; i.e., germicide registered by EPA effective against Salmonella choleraesuis, Staphylococcus aureus, and Pseudomonas aeruginosa for use on nonliving objects in dental and medical facilities
- Compatibility with surfaces in your facility
- Cleaning as well as disinfecting properties (manage inventory)
- Low allergenicity for both patients and dental team
- Ease of use -- ready to use or mixing required
- Clear, easy-to-follow instructions
- A reasonable contact time; i.e., 10 minutes or less
- Acceptable storage and disposal requirements
- Reasonable use life and shelf life
Details about common disinfectants
- CaviCide1 by TotalCare has 17.2% isopropanol with 0.28% Quats as their active ingredients. CaviCide1 is EPA registered, has a TB claim of one-minute kill time. This product is an intermediate-level disinfectant. CaviCide1 nonwoven wipes may be used for cleaning as well as disinfection. Wipes are available in a variety of sizes, 6X6.75", FlatPack 7x9 and 9x12. This product is available in flat packs and canisters. Features are fast-acting kill times against tuberculosis, methicillin-resistant Staphylococcus aureus, HIV, and hepatitis B and C. For more information, visit www.totalcareprotects.com.
- DisCide ULTRA wipes by Palmero Healthcare have active ingredients of quaternary ammonium <1% and isopropyl alcohol 63.25%. DisCide Ultra is EPA-registered, with a TB claim of one-minute kill time. This product is a hospital-level, one-step, ready-to-use, high-level alcohol-based disinfectant. DisCide Ultra kills TB, MRSA, HIV, H1N1, HBV, HBC, VRE, RSV, H3N2, influenza A, adenovirus, HSV-2, coronavirus, Pseudomonas aeruginosa, salmonella, staph, E. coli, athlete's foot, and a host of others microorganisms in one minute or less. DisCide ULTRA wipes are a generous size of 10.5" x 10.5" with 60 wipes per canister. For more information, visit www.palmerohealthcare.com.
- OPTIM 33 TB wipes by SciCan have active ingredients of 0.5% accelerated hydrogen peroxide. OPTIM 33 TB is EPA-registered, with a TB claim of five minutes. This intermediate-level disinfectant is a water based, odorless, one-step cleaner. OPTIM 33 TB has no volatile organic compounds. OPTIM 33 TB kills Mycobacterium TB, human coronavirus (SARS), HIV-1, hepatitis B, hepatitis C, avian influenza (bird flu), Salmonella, E. coli, Pseudomonas, Staphylococcus aureus, MRSA, and other viruses and bacteria. In addition, OPTIM 33 TB achieves 99.99% broad-spectrum kill of vegetative bacteria in 30 seconds. OPTIM 33 TB is available in liquid and wipes. Accelerated hydrogen peroxide has been blended with commonly used safe ingredients. OPTIM 33 TB has no volatile organic compounds and is safe for the environment. For more information, visit www.SciCan.com.
- Opti-Cide3 Wipes by Biotrol are EPA-registered, ready-to-use, disinfectant cleaner with a TB claim of three minutes. Opti-Cide3 has active ingredient of dimethylbenzylammonium chloride.
This intermediate-level disinfectant and cleaner provides a broad spectrum disinfectant that effectively kills the following microorganisms at room temperature (69°F/20°C) with a three-minute contact time: Mycobacterium bovis BCG (tuberculosis), Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella choleraesuis, Trichophyton mentagrophytes, hepatitis B virus (HBV), hepatitis C virus (HCV), rhinovirus, polio I virus. Opti-Cide3 has a family of products which include a spray bottle for spray-wipe-spray method. Each container of wipes contains 100 7" x 10" wipes. Please read label for more instructions. For more information, visit www.biotrol.com.
- PDI Sani-Cloth AF3 by Crosstex is an EPA-registered, ready-to-use, alcohol-free disinfectant wipe. PDI Sani-Cloth AF3 active ingredients include N-alkyldimethylbenzylammonium chloride. PDI Sani-Cloth is fragrance free with a kill time of three minutes for all TB and microorganisms. Sani-Cloth has been tested on 44 microorganisms. Instructions for this product are available in English and Spanish. Sani-Cloth canisters include 160 large (6" x 6.75") or 65 extra large wipes (7.5" X 15.0"). What looks visibly wet on one surface type may look different on another surface type. Wall chart for instructions is available. Visit www.crosstex.com for more information.
- ProSpray Wipes by Certol International are a ready-to-use, EPA-registered disinfectant with a TB claim of 10 minutes. This intermediate-level disinfectant cleaner has a water based, nonalcohol formula with lemon scent and built-in surfactants, approved for use in both medical and dental settings. The active ingredients are o-phenylphenol (OPP) and o-benzyl-p-chlorophenol (OBCP). This broad-spectrum, water-based antimicrobial disinfectant has the same reliable formula for both wipes and liquids and can be used on a variety of surfaces, telephones, computers, and other electronic equipment. These wipes have antimicrobial activity against Staphylococcus aureus, HIV, TB, and many other organisms. ProSpray surface cleaner and disinfectant contains detergents and surfactants for essential precleaning. This formula sustains antimicrobial action after application, which is not found with alcohol or bleach compounds. For more information, visit www.certol.com.
The choice of disinfectant, concentration, and exposure time is based on the risk for infection associated with use of equipment and other factors discussed.
Past RDH Issues