Proliferative verrucous leukoplakia
by Nancy W. Burkhart, RDH, EdD
[email protected]
Your patient is Thomas, who is 56 years old. He is seeking treatment for white, plaque-like lesions along the gingival margins of the maxillary teeth with a clinical diagnosis of leukoplakia. Thomas has observed the tissue in its present state for a few months and is concerned that it may be cancer. He is a nonsmoker with an insignificant health history. Leukoplakia is a clinical term that is based on a World Health Organization definition: “The term leukoplakia should be used to recognize white plaques of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer.” (Warnakulasuriya et al. 2007)
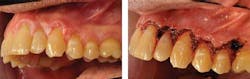
A differential diagnosis was made in this case with a possibility of proliferative verrucous leukoplakia (PVL) based on the clinical appearance and lack of a firm etiology (see Figure 1). A biopsy was performed and a diagnosis of benign hyperkeratosis without dysplasia was rendered.
Although the diagnosis was not proliferative verrucous leukoplakia (PVL) at this time, early stages of the disease have a very similar clinical appearance, and PVL is known to progress through stages from hyperkeratosis to dysplasia followed by squamous cell carcinoma. Although the etiology of PVL is not known, it was first described in 1985, and is a particularly aggressive, persistent, and irreversible form of leukoplakia.
Plaque-like lesions appear as flat hyperkeratotic tissue but exhibit persistent growth becoming exophytic and verrucous in nature. Additionally, PVL exhibits a tendency to form on the keratinized gingival tissue, retromolar pads, the palate, buccal mucosa, and the tongue. It is known to progress to squamous cell carcinoma (SCC) in most cases. The lesions are slow growing, have a recognized phenomenon known as field cancerization (appearance of multiple primary tumors) and exhibit a high rate of recurrence after treatment.
Reported cases by Silverman, Jr. et al. 1997, found PVL more frequently in women with a 4 to 1 ratio in a group of 54 patients with a mean age of 62 years old. The buccal mucosa was the most common site in women and the tongue in men. The tongue and the gingiva were also found to have the greatest tendency toward malignancy. Similar reports by Bagan et al. 2007 support a female predilection, gingival propensity, and a mean age of 70.
Laser surgery was performed in this case to remove the long-standing questionable tissue and to prevent further progression of any diseased tissue (see Figure 2). The white plaques are clinically indistinguishable from any typical leukoplakia, but they do exhibit a verrucous texture in most cases. Reported cases of early hyperkeratosis with progression to PVL are documented in the literature.
Studies suggest that this disease is progressive and exhibits a high rate of recurrence and malignant transformation to squamous cell carcinoma in the range of 70%. The reported time from the diagnosis of PVL to squamous cell carcinoma has been documented from 4.4 years (Bagan et al. 2003) to 11.6 years’ duration (Silverman and Gorsky, 1997).
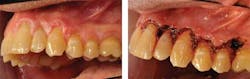
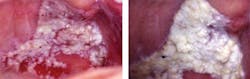
PVL of the gingiva has been suggested as a subset of proliferative verrucous leukoplakia (Fettig et al. 2009), targeting the specific tissue of the gingiva. Both the free and attached gingiva have been reported in these cases of PVL. Figures 3 and 4 depict a patient who was diagnosed with PVL (Figure 3) and suffered a recurrence of PVL (Figure 4) that was even more intense, with subsequent development of SCC.
Relationship to SCC
Although no etiology is known for PVL, several findings have been suggested in reported studies related to squamous cell carcinoma. Human papillomavirus (HPV) 16 and 18 have been correlated with head and neck cancers. HPV 16 has been correlated with several types of oral cancers and specifically tongue and oropharyngeal cancer. Epstein-Barr virus has been documented to be an agent in nasopharyngeal carcinoma and associated with oral hairy leukoplakia, lymphoproliferative disease, B-cell lymphomas, and lymphoepithelial carcinoma.
Bagan et al. in 2008 reported EBV in 60% of a subgroup of patients with PVL. The authors reported EBV in 40% of a subgroup of patients with oral squamous cell carcinoma and 0% in a control group.
Morton et al. in 2007 stated that the cellular characteristics involving the lymphocytic infiltrate seen in the histological diagnosis of PVL may be mistaken for lichen planus in some cases. The authors state, “The lymphocytic infiltrate may be intense and focally obscure visualization of the basement membrane and the epithelial connective tissue interface.”
Treatment
The treatment that is recommended consists of multiple techniques such as CO2 laser, surgery, surgery associated with radiotherapy, cryotherapy, retinoid A, systemic vitamin A therapy, topical vitamins, bleomycin, and photodynamic therapy. Any leukoplakia type lesions should be assessed, documented, and followed long term for recurrence.
Early treatment results in less tissue loss, better results, and a wider range of options.
Discussion
Gandolfo et al. in 2009 suggested that periodontists should be cognizant of PVL since periodontal sites are frequently involved in this disease. The same suggestion should be made for the role of dental hygienists since their treatment is primarily involved in assessing these sites as well.
Early recognition, referral, and treatment are key factors in oral cancer prevention and (early) detection. Hygienists are in a position to note and monitor mucosal changes of the gingiva and all oral tissues. Early detection should be a goal for all dental professionals at each recall visit, consisting of careful evaluation and monitoring of the tissue. Bagan et al. in 2009 stated, “The primary objective of leukoplakia therapy should be the prevention of malignant transformation.”
Subtle changes in the oral tissues may be documented using digital photography and careful documentation. Tissue that appears to progress in ulceration or texture, exhibits a nonhealing characteristic, and tissue damage such as leukoplakia without etiology need to be called into question. If no change is observed or no cause identified, a biopsy should be performed and/or referral to an oral medicine specialist should occur. Additionally, future biopsies should be performed when changes occur on previously biopsied tissue or surgically removed lesions that may indicate a progression of the disease or possibly a new disease entirely that has occurred.
Since 1997, I have moderated the International Oral Lichen Planus Support Group. I have heard from many patients, practitioners, and researchers from all over the world regarding the diagnosis and treatment of lichen planus. One of the prime problems that I have observed in patients who have subsequently developed oral cancer is the fact that many lesions are monitored for years and the original biopsy is the diagnosis that is attached to the patient’s complaints. Many patients report that they were told, “It is only lichen planus — learn to live with it.”
Somewhere in the interim, a change occurred in the tissue that may have been diagnosed with a new biopsy and an objective reassessment of the chief complaint. Many times that original disease state has changed and we are dealing with a new set of problems. This is probably true for PVL as well. Although the case presented here was hyperkeratosis, the literature supports the fact that — given the clinical representation and the lack of a determined etiology — the tissue may be in an early stage of PVL.
Any leukoplakia lesion should be assessed and treated. Any lesion that has been removed should be reassessed consistently for any changes or recurrence. Digital photography is excellent for future comparison. Early detection of oral cancer is the treatment of choice and you could be the person who saves a life. Keep listening to your patients and asking good questions! You are in a prime position to help patients maintain both oral and general health.
References
Bagan JV, Jimenez Y, Murillo J, Poveda R, Diaz JM, Gavalda C, Margaix M, Scully C, Alberola TM, Puente MT, Alonso MP. Epstein-Barr virus in oral proliferative verrucous leukoplakia and squamous cell carcinoma: A preliminary study. Med Oral Patol Oral Cir Bucal 2008 Feb1:13(2):E110-3.
Bagan JV, Jimenez Y, Sanchis JM, Poveda R, Millan MA, Murillo J, Scully C. Proliferative verrucous leukoplakia: High incidence of gingival squamous cell carcinoma. J Oral Pathol Med 2003;32:379-382.
Cohan DM, Popat S, Kaplan SE, Rigual N, Loree T, Hicks WL. Oropharyngeal cancer: current understanding and management. Current Opinion in Otolaryngology & Head and Neck Surgery. 2009, 17:88-94.
Fettig A, Pogrel MA, Silverman S, Bramanti TE, Da Costa M, Regezi JA. Proliferative verrucous leukoplakia of the gingiva. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod 2000; 90(6):723-30.
Gandolfo S, Castellani R, Pentenero M. Proliferative verrucous leukoplakia: a potentially malignant disorder involving periodontal sites. J Periodontol. 2009 Feb;80(2):274-81.
Morton TH, Cabay RJ, Epstein JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: report of three cases. J Oral Pathol Med. 2007 May;36(5):315-8.
Poveda-Roda R, Bagan JV, Jimenez-Soriano Y, Diaz-Fernandez JM, Gavalda-Esteve C. Retinoids and proliferative verrucous leukoplakia (PVL). A preliminary study. Med Oral Pathol Oral Cir Bucal. 2010 Jan 1:15(1):e3-9.
Silverman S, Gorsky M. Proliferative verrucous leukoplakia: A follow-up study of 54 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997: 84:154-57.
Warnakulasuriya S, Johnson NW, van der Wall L. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007 36:575-80.
Figure 1: Patient presents with clinical characteristics of PVL (courtesy of Dr. Thomas Caputo). Figure 2: Laser treatment of the affected area seen in Figure 1 (courtesy of Dr. Thomas Caputo). Figure 3: Proliferative verrucous leukoplakia before removal (courtesy of Dr. T.D. Rees). Figure 4: PVL after removal as seen in Figure 3. Recurrence with intense exophytic lesions during later diagnosis of SCC (courtesy of Dr. T.D. Rees).
About the Author
Nancy W. Burkhart, BSDH, EdD, is an adjunct associate professor in the department of periodontics, Baylor College of Dentistry and the Texas A & M Health Science Center, Dallas. Dr. Burkhart is founder and co-host of the International Oral Lichen Planus Support Group http://www.bcd.tamhsc.edu/outreach/lichen/ and coauthor of General and Oral Pathology for the Dental Hygienist. Her Web site for seminars is www.nancywburkhart.com.