Listen to the article on our podcast!
While oral squamous cell carcinoma (OSCC) remains the most frequently discussed oral malignancy, numerous other cancers can arise within the oral cavity.1 Although these tumors occur less commonly than OSCC, they present distinct diagnostic and therapeutic challenges. This article surveys several of these malignancies—from salivary gland tumors to sarcomas—to highlight their presentations, pathology, and management.
Salivary gland tumors
Though relatively rare, salivary gland tumors can exhibit remarkable histological diversity since they originate in the parotid, submandibular, sublingual, or minor salivary glands.2 The 2022 newest World Health Organization classification of head and neck tumors acknowledges newly recognized entities such as microsecretory carcinoma.3 However, despite these updates, the most frequently diagnosed malignant subtypes remain mucoepidermoid carcinoma, acinic cell carcinoma, adenoid cystic carcinoma, carcinoma ex-pleomorphic adenoma, and adenocarcinoma.
Clinically, salivary gland tumors often first present as localized swelling, sometimes accompanied by peripheral facial nerve dysfunction, skin fixation or invasion, and lymph node involvement in the neck. The initial diagnosis typically relies on ultrasound-guided fine-needle aspiration cytology, a valuable tool for detecting malignancy. However, this method has limitations in accurately determining the tumor’s specific subtype and grade.
Mucoepidermoid carcinoma (MEC) is the most common malignant salivary gland tumor, accounting for 10%–15% of all salivary gland neoplasms and approximately 30% of salivary malignancies.4 It presents as a slow-growing, painless swelling, most commonly affecting the palate, with a higher prevalence in females. Treatment primarily involves surgical excision, while radiation therapy may be considered for high-grade tumors or cases with close surgical margins.
Acinic cell carcinoma (ACC) is a low-grade malignant tumor commonly found in the parotid gland. It will appear as a slow-growing, painless mass and is more frequently observed in women. Risk factors include prior radiation exposure and potential genetic predisposition. Diagnosis is based on imaging studies and often requires a biopsy (fine-needle aspiration or surgical excision). Primary treatment is surgical resection, with radiation therapy reserved only for high-risk cases, such as those with aggressive features, positive margins, lymph node involvement, or when surgery is not an option.5 Although ACC generally carries a favorable prognosis, it has the potential for recurrence and distant metastasis, most commonly to the lymph nodes and lungs, necessitating long-term follow-up.
Adenoid cystic carcinoma is known for its tendency to grow along nerves, which can result in considerable pain and may even lead to facial paralysis. It most commonly originates in the palate (specifically within the mucous-secreting minor salivary glands). It only accounts for about 1% of all malignant head and neck tumors and approximately 10% of salivary gland cancers. While it is most common in people in their 50s and 60s, it can occur at any age, with a mild female predominance. ACC presents as a slow-growing mass that can also be found in the tongue, paranasal sinuses, nasopharynx, and lacrimal glands.6 Histologically, it exhibits a distinct cribriform or “Swiss cheese” pattern. Due to its highly infiltrative nature, treatment typically involves wide surgical excision, often followed by postoperative radiation therapy.7,8
Oral melanoma
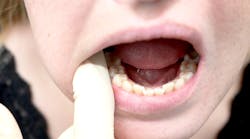
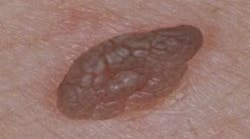
Oral melanoma is a rare and highly aggressive cancer that comprises about 0.5% of oral malignancies and 1%–2% of all melanomas. It frequently presents as a pigmented lesion on the palate, gingiva, or buccal mucosa, but some lesions could be amelanotic, which complicates timely recognition.9 Therefore, late detection contributes to a poor five-year survival rate of approximately 12.3%. Diagnosis rests on biopsy and immunohistochemical staining for markers such as S-100 and HMB-45. The treatment centers on wide surgical excision, often combined with additional therapies such as radiotherapy, chemotherapy, or immunotherapy for advanced cases.10
Lymphoid neoplasms
Lymphomas in the oral cavity and oropharynx are uncommon, accounting for around 2%–5% of oral malignancies. Most involve non-Hodgkin’s lymphomas (NHL), especially in Waldeyer’s ring (tonsils and base of the tongue), where lymphoid tissue is abundant.
Diffuse large B-cell lymphoma (DLBCL) is the most frequent aggressive subtype, often presenting as a rapidly enlarging mass or ulcer, occasionally accompanied by fever, night sweats, and weight loss.
Burkitt’s lymphoma is another highly aggressive form, typically linked to Epstein-Barr virus (EBV) in its endemic manifestation and often affecting the jaw or maxilla in children.
Less aggressive, “indolent” variants, such as follicular lymphoma or extranodal marginal zone B-cell lymphoma (MALT lymphoma), can remain undetected for longer periods yet have the potential to transform into more aggressive forms over time.11
Hodgkin’s lymphoma, defined by Reed-Sternberg cells, rarely originates in the oral region. At the same time, T-cell and NK/T-cell lymphomas can appear here but tend to be particularly aggressive and destructive. Diagnosis typically requires a biopsy with immunohistochemical markers to determine lineage, alongside imaging to establish disease extent. Treatment primarily relies on systemic therapies, chemotherapy, and targeted agents (such as rituximab for B-cell cancers)—with radiotherapy used selectively and surgery generally reserved for diagnostic or palliative purposes. Prognosis depends on tumor subtype, disease stage, and patient factors, underscoring the importance of early recognition and prompt treatment, even when oral lesions are subtle.12-14
Sarcomas
Sarcomas are malignant tumors derived from mesenchymal tissues.
Kaposi’s sarcoma is a malignant vascular tumor most often linked to human herpesvirus 8 (HHV-8). It is most commonly seen in individuals with advanced HIV/AIDS (epidemic KS). However, it can also occur in other contexts, such as classic (Mediterranean) KS, iatrogenic (transplant-related) KS, and endemic (African) KS. The form associated with HIV infection is by far the most prevalent in the oral cavity, where the weakened immune system allows the HHV-8 virus to replicate unchecked.15
Clinically, it presents as reddish-purple or bluish-black macules, plaques, or nodules, most frequently affecting the hard palate, gingiva, or dorsal tongue. Lesions can be asymptomatic, but as they enlarge, they may ulcerate or bleed, causing discomfort and interfering with speech or eating. Diagnosis is made based on clinical evaluation, biopsy, and histopathological examination. An HIV test is crucial in patients with an unknown immunocompromised status.
Management depends on disease extent, immune status, and symptom severity. In HIV-positive individuals, highly active antiretroviral therapy (HAART) is essential to improve immune function, which can stabilize or even induce regression of KS lesions. Local therapies, such as surgical excision, laser ablation, or intralesional chemotherapy, may help control solitary or symptomatic lesions. More widespread disease often requires systemic chemotherapy or targeted therapies (e.g., interferon-alpha or newer immunomodulatory agents). Prognosis varies significantly, with localized disease faring better than disseminated disease in immunocompromised patients.
Osteosarcoma arises from mesenchymal cells capable of producing osteoid and is most frequently seen in the long bones of adolescents. Craniofacial osteosarcomas represent about 6%–8% of cases and are most often found in the mandible or maxilla. Risk factors include prior radiation, Paget’s disease, and certain hereditary syndromes.16
Clinically, symptoms range from swelling and pain to tooth mobility due to rapid jaw expansion. Panoramic x-rays and CT scans typically reveal bone destruction or periosteal reactions, but a biopsy is always required for a definitive diagnosis. The treatment involves wide surgical excision with clear margins and adjuvant chemotherapy, and radiation therapy may be an option in inoperable cases or when margins are not fully clear. A five-year survival rate can reach 50%–70% for localized disease, although outcomes depend on tumor stage and response to chemotherapy.17
What we need to know (and do)
We should maintain a high level of suspicion for any persistent or atypical lesion in the oral cavity. Early steps include:
Thorough clinical examination by carefully inspecting and palpating all intraoral tissues.
Use x-rays, CT, MRI, or PET scans to assess the lesion’s extent and rule out metastatic disease.
Don’t hesitate to refer to a specialist. A biopsy is the definitive diagnostic tool. Histopathological and immunohistochemical analyses determine tumor type and guide treatment.
And here’s a startling revelation: while mastering the spectrum of primary oral malignancies is essential, it’s equally vital to remember that the oral cavity can sometimes become a rare battleground for metastatic tumors from distant sites, most notably breast, lung, and kidney.17 These secondary tumors commonly appear as fast-growing masses and may have variable clinical aspects depending on their location; they may be the first sign of underlying cancer elsewhere,18 an “aha” moment that underscores the importance of comprehensive diagnostic vigilance.
A final word
Malignancies beyond OSCC, while less common, often necessitate specialized expertise due to their diverse nature and potential for aggressive behavior. Diagnostic delays remain a significant concern, frequently stemming from limited awareness and the capacity of these tumors to mimic benign conditions. To address this, we must embrace advancements in diagnostic imaging, explore the potential of more sensitive biomarkers, and critically prioritize continuing education for dental professionals.
Acknowledging that OSCC is not the sole malignant threat within the oral cavity creates a heightened sense of clinical vigilance. By integrating cutting-edge technologies, refining our diagnostic skills, and cultivating robust interprofessional relationships, we can strive for earlier detection, ultimately improving outcomes for patients facing these rare yet equally impactful oral cancers. Our commitment to comprehensive patient care demands nothing less.
Editor's note: This article appeared in the April/May 2025 print edition of RDH magazine. Dental hygienists in North America are eligible for a complimentary print subscription. Sign up here.
References
- Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491-508. doi:10.1016/j.soc.2015.03.006
- Young A, Okuyemi OT. Malignant Salivary Gland Tumors. StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK563022/
- Skálová A, Hyrcza MD, Leivo I. Update from the 5th edition of the World Health Organization Classification of Head and Neck Tumors: Salivary Glands. Head Neck Pathol. 2022;16(1):40-53. doi:10.1007/s12105-022-01420-1
- Peraza A, Gómez R, Beltran J, Amarista FJ. Mucoepidermoid carcinoma. An update and review of the literature. J Stomatol Oral Maxillofac Surg. 2020;121(6):713-720. doi:10.1016/j.jormas.2020.06.003
- Al-Zaher N, Obeid A, Al-Salam S, Al-Kayyali BS. Acinic cell carcinoma of the salivary glands: a literature review. Hematol Oncol Stem Cell Ther. 2009;2(1):259-264. doi:10.1016/s1658-3876(09)50035-0
- Romanò R, De Felice F, Ferri A, et al. Adenoid cystic carcinoma of minor salivary glands (AdCCmSG): a multidisciplinary update. Expert Rev Anticancer Ther. 2024;24(7):567-580. doi:10.1080/14737140.2024.2357806
- Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: a review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38(4):620-627. doi:10.1002/hed.23925
- Cleymaet R, Vermassen T, Coopman R, Vermeersch H, De Keukeleire S, Rottey S. The therapeutic landscape of salivary gland malignancies–where are we now? Int J Mol Sci. 2022;23(23):14891. doi:10.3390/ijms232314891
- Lamichhane NS, An J, Liu Q, Zhang W. Primary malignant mucosal melanoma of the upper lip: a case report and review of the literature. BMC Res Notes. 2015;8:499. doi:10.1186/s13104-015-1459-3
- Bhullar RP, Bhullar A, Vanaki SS, Puranik RS, Sudhakara M, Kamat MS. Primary melanoma of oral mucosa: a case report and review of literature. Dent Res J (Isfahan). 2012;9(3):353-356.
- Flores-Hidalgo A, Bankhead A, Murrah V, Padilla R. Malignant lymphoproliferative disorders of the oral and maxillofacial region: report of two institutions. Front Oral Health. 2022;3:802555. doi:10.3389/froh.2022.802555
- Abdelwahed Hussein MR. Non-Hodgkin’s lymphoma of the oral cavity and maxillofacial region: a pathologist viewpoint. Expert Rev Hematol. 2018;11(9):737-748. doi:10.1080/17474086.2018.1506326
- Laskar S, Mohindra P, Gupta S, Shet T, Muckaden MA. Non-Hodgkin lymphoma of the Waldeyer’s ring: clinicopathologic and therapeutic issues. Leuk Lymphoma. 2008;49(12):2263-2271. doi:10.1080/10428190802493686
- Silva TD, Ferreira CB, Leite GB, de Menezes Pontes JR, Antunes HS. Oral manifestations of lymphoma: a systematic review. Ecancermedicalscience. 2016;10:665. doi:10.3332/ecancer.2016.665
- Facciolà A, Venanzi Rullo E, Ceccarelli M, et al. Kaposi’s sarcoma in HIV-infected patients in the era of new antiretrovirals. Eur Rev Med Pharmacol Sci. 2017;21(24):5868-5869. doi:10.26355/eurrev_201712_14036
- Alramadhan SA, Vyas R, Cohen DM, Bhattacharyya I, Islam MN, Reith JD. An unusual histopathological presentation of mandibular osteosarcoma. Head Neck Pathol. 2024;18(1):121. doi:10.1007/s12105-024-01724-4
- Kaimal VG, Pai A. Osteosarcoma of mandible – a case report. Indian J Surg Oncol. 2024;15(3):495-498. doi:10.1007/s13193-024-01933-x
- Bodner L, Sion-Vardy N, Geffen DB, Nash M. Metastatic tumors to the jaws: a report of eight new cases. Med Oral Patol Oral Cir Bucal. 2006;11(2):E132-E135.