Sugar free
Sugar-free foods and beverages can assist consumers in maintaining optimal oral health
Polyols, or sugar alcohols, are more and more frequently used to replace sugars in a variety of foods and beverages. They have been used in sugar-free gums and candies for many years, and since the “low carb” craze, they are commonly found in a wider variety of products. Polyols are reduced in calories, have a reduced impact on blood glucose levels compared to sugars, and do not promote dental caries.
Polyols serve as useful alternatives to sugars in products such as chewing gums, candies, ice cream, baked goods, and fruit spreads. They also function well in fillings and frostings, canned fruits, beverages, yogurt, and tabletop sweeteners. Polyols are commonly used in toothpastes, mouthwashes, and pharmaceutical products such as cough syrups and throat lozenges. The polyols currently available for use in the United States are erythritol, polyglycitols (including maltitol syrups), isomalt, lactitol, maltitol, mannitol, sorbitol, and xylitol.
The terms polyols and sugar alcohols are being used here interchangeably although the U.S. Food and Drug Administration now requires use of the term sugar alcohols on the food label.1 This term is very confusing to consumers since sugar alcohols are not sugars and they are not alcohols. Even if the consumer is familiar with the term sugar alcohol, she/he is unlikely to know what a sugar alcohol is.
Polyols are a group of low-digestible carbohydrates derived from the hydrogenation of their sugar or syrup source (e.g., sorbitol from glucose, lactitol from lactose). Since the term “sugar alcohol” will probably only appear on the food label when a nutrient claim such as “sugar-free” is made, the consumer may indeed be confused when the term “sugar alcohol” also appears on the label. A nationally projectable consumer survey found that 78 percent of those surveyed think the term “sugar alcohol” indicates a product contains some sugar even when the product is labeled “sugar-free.” Sixty-nine percent believe the product contains some alcohol. A petition has been submitted to the FDA to allow use of the term polyols in lieu of sugar alcohols on the food label.2
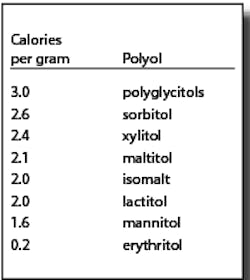
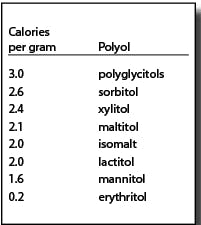
Polyols are not metabolized like sugars. They are generally incompletely absorbed. The absorbed portions are either metabolized (generally by insulin-independent mechanisms) or excreted via urine. A significant amount of the unabsorbed portion is metabolized to short chain fatty acids and gases by gut microflora.3 Due to their different metabolic fates, polyols provide fewer than the traditional four calories per gram for other carbohydrates. The FDA allows the use of the caloric values in the chart at right:4
As a result of their incomplete absorption and method of metabolism, overconsumption of polyol-containing foods may, in some people, result in gastrointestinal symptoms, including laxative effects; similar to reactions following the consumption of beans, cabbage, and certain high-fiber foods. Such symptoms are dependent upon a person’s sensitivity and the other foods eaten along with the polyol-containing product. Any gastrointestinal symptoms (such as a feeling of fullness) from consuming foods with polyols, if they occur at all, are usually mild and temporary. Most people adapt to polyols after a few days the same way they do to high-fiber foods. Food manufacturers advise consumers of these possible effects through product labeling.
Also related to the manner in which they are metabolized, polyols do not cause sudden increases in blood glucose levels; therefore, they are recognized as being useful in the diets of those with diabetes. For example, the American Diabetes Association recognizes that polyols “produce a lower postprandial response than fructose, sucrose, or glucose and have lower energy values.”5
Clinical studies have demonstrated a consistent decrease in dental caries, ranging from 30 to 60 percent, among subjects using sugar replacers as compared to subjects in a control group.6 Some polyols are not as sweet as sugar and may be used in combination with other polyols and/or approved low-calorie sweeteners.
The noncariogenic property of polyols and their benefit to oral health have been recognized in many countries including the United States. Terms such as “does not promote tooth decay” and “may reduce risk of tooth decay” have long been used on “sugar-free” chewing gums and candies sweetened with these sugar replacers. In the United States, these products can only be labeled “sugar-free” if the food contains less than 0.5 grams of sugar per reference amount. “Sugar” includes only mono- and disaccharides. Foods sweetened with polyols or polyols in combination with low-calorie sweeteners may be described as “sugar-free.” If a “sugar-free” product does not qualify as being low-calorie or reduced-calorie, it must bear a disclaimer that it is not for weight control.7
The FDA has authorized the use of a “does not promote tooth health decay” claim on sugar-free foods and beverages containing xylitol, sorbitol, mannitol, maltitol, isomalt, lactitol, polyglycitols, maltitol syrups, erythritol or a combination of these. Products sweetened with the novel sugar D-tagatose may also bear the health claim but may not be labeled sugar-free.
The health claim regulation provides that when carbohydrates other than those listed above are present in the food, “the food shall not lower plaque pH below 5.7 by bacterial fermentation either during consumption or up to 30 minutes after consumption, as measured by the indwelling plaque test found in ‘Identification of Low Caries Risk Dietary Components,’ dated 1983, by T.N. Imfeld, Volume 11, Monographs in Oral Science.” Furthermore, FDA regulations prohibit the expansion of the health claim beyond the parameters set by the FDA, and the claim may not attribute any degree of the reduction of risk of dental caries to the use of the noncariogenic-carbohydrate-sweetener-containing food.8 The claim may not imply that consuming noncariogenic-carbohydrate-sweetener-containing foods is the only recognized means of achieving a reduced risk of dental caries.
In October 1998, the American Dental Association’s House of Delegates approved a position statement acknowledging the “Role of Sugar-Free Foods and Medications in Maintaining Good Oral Health.” The ADA recognizes that “it is neither advisable nor appropriate to eliminate from the American diet sugar-containing foods that provide necessary energy value for optimal nutrition.” The Association strongly recommends, however, “that major efforts be made to promote the use of sugar-free foods or chewing substances in place of sugar-containing foods that involve a frequent intake or repeated oral use ... use of these sugar-free products will contribute to improved oral health.”9
The United States and numerous other countries as well as international regulatory and health agencies have affirmed the safety of polyols. Canada allows for the use of erythritol, isomalt, lactitol, maltitol, maltitol syrup, mannitol, polyglycitols, sorbitol, and xylitol, whereby the maximum level of their use in unstandardized food is limited only by good manufacturing practices.10 Additionally, they have been carefully reviewed by the Joint Expert Committee on Food Additives (JECFA) of the United Nations’ Food and Agriculture Organization and the World Health Organization and deemed safe. JECFA has assigned an acceptable daily intake or ADI of “not specified,” the most favorable assignation, to each of the polyols. An ADI “not specified” means that, on the basis of the available data, the total daily intake of the substance arising from its use at the levels necessary to achieve the desired effect and from its acceptable background in food does not, in the opinion of the Committee, represent a hazard to health.11-15
Recent consumer research found that 84 percent of American adults use sugar-free foods and beverages regularly. This is up from 73 percent in 1998. The same survey found that 35 percent of adults say they are more likely to buy a sugar-free candy or gum if it is labeled “does not promote tooth decay.”16 With this increased consumer interest in low-calorie, sugar-free products, as well as the increased availability of polyols and innovations in food technology, additional good-tasting sugar-free products bearing the “does not promote tooth decay” health claim are expected to be available. These products can assist consumers in maintaining good oral health.
Lyn O’Brien Nabors is the executive vice president of the Calorie Control Council, an international association of manufacturers and suppliers of sugar-free, fat-free, and light foods and beverages.
References
1 Office of the Federal Register, General Service Administration, 2004. Code of Federal Regulations. Title 21, Section 101.9. Revised as of April 1, 2004. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.9.
2 Calorie Control Council request to amend food labeling regulations to permit the use of the term polyols in lieu of sugar alcohols in the Nutrition Facts Panel on the food label. 1995. FDA Docket No. 95P0099.
3 Livesey G. Health potential of polyols as sugar replacers with emphasis on low-glycemic properties. Nutr Res Rev 2003; 163-191.
4 Federation of American Societies for Experimental Biology (FASEB), Life Sciences Office. The evaluation of the energy of certain sugar alcohols used as food ingredients. Bethesda, Md.: FASEB; 2004 (unpublished).
5 American Diabetes Association. Position Statement. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2002; 25(1), 202-212.
6 Hayes C. The effect of noncariogenic sweeteners on the prevention of dental caries: A review of the evidence. J Dental Ed 2001; 65:1106-1109.
7 Office of the Federal Register, General Service Administration, 2004. Code of Federal Regulations. Title 21, Section 101.60. Revised as of April 1, 2004. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.60.
8 U.S. Food and Drug Administration, Fed. Reg. 68:39831. July 3, 2003.
9 American Dental Association. Position Statement. Role of Sugar-Free Foods and Medications in Maintaining Good Oral Health. Adopted October 1998. http://www.ada.org/prof/resources/positions/statements/sugarfre.asp.
10 Canada Gazette Part II, 1993. Food and Drug Regulations, amendment, Vol. 127, No. 12, pp. 2601-2608. Ottawa, June.
11 FAO/WHO Expert Committee on Food Additives. 1989. Thirty-third Report, “Evaluation of certain food additives and contaminants,” Maltitol and Maltitol Syrup. WHO Technical Report Series, No. 776, pp. 19-20.
12 FAO/WHO Expert Committee on Food Additives. 1987. Thirtieth Report, Mannitol. WHO Technical Report Series, No. 751, pp. 25-26, 52.
13 FAO/WHO Expert Committee on Food Additives. 1985. Twenty-ninth Report, “Toxicological evaluation of certain food additives and contaminants,” Isomalt. WHO Food Additive Series, No. 20, pp. 207-237.
14 FAO/WHO Expert Committee on Food Additives. 1983. Twenty-seventh Report, “Toxicological evaluation of certain food additives and contaminants,” Xylitol and Lactitol. WHO Technical Report Series, No. 696, pp. 82-94, 161-174.
15 FAO/WHO Expert Committee on Food Additives. 1982. Twenty-sixth Report, “Toxicological evaluation of certain food additives and contaminants,” Sorbitol. WHO Technical Report Series, No. 683, pp. 218-228.
16 Calorie Control Council. 2004. Light Products and Weight Control Habits Survey (unpublished).