New treatment for sleep apnea may replace bulky CPAPs for patients
Listen to the article on our podcast!
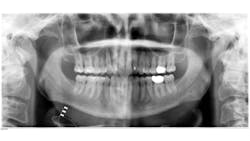
As a dental hygienist, if you saw this panoramic image, what would you think?
One afternoon in private practice, I took a panoramic radiograph like any other day. When evaluating the radiograph, I noticed a radiolucent wiring that confused me. I didn’t remember my patient having a history of any oral surgeries that would indicate this.
The patient informed me that the wire I was seeing was a new device to treat his sleep apnea. The patient then asked if I had ever heard of Inspire. I said no and felt humbled. As a dental hygiene educator, I like to think I am up-to-date on the latest oral health news, but this new device for the treatment of sleep apnea was news to me! I hope I can educate my fellow oral health colleagues on this device and how it could be a life-changing solution for those with moderate to severe sleep apnea.
The effects of OSA
According to the National Council on Aging, obstructive sleep apnea (OSA) occurs in more than 39 million adults in the United States.¹ The Centers for Disease Control and Prevention found that three in four US adults have sleep disorder symptoms.1 OSA occurs when the upper airway becomes blocked during sleep, interfering with breathing.
Signs and symptoms of OSA are loud snoring, gasping for air during sleep, daytime sleepiness, dry mouth, headache, and frequent waking. OSA is a concern for all health professionals as it can increase the risks for health conditions such as type 2 diabetes, high blood pressure, heart failure, kidney disease, stroke, fatty liver disease, and sexual dysfunction.
The gold standard for diagnosing OSA is through laboratory polysomnography,2 which is used during a sleep study. For many Americans, the typical prescribed therapy is a continuous positive airway pressure (CPAP) machine at night to mitigate the health risks associated with sleep apnea.3 Adherence to recommended uses of these bulky devices is difficult, however, and can interfere with optimal OSA management.3
A new device to treat sleep apnea
A new device by Inspire could increase patient compliance and better manage OSA. Inspire therapy is the only FDA-approved (in 2014) OSA therapy that works comfortably inside the body.4 The small Inspire implant delivers gentle pulses to the airway muscles to keep the airway open so the patient can breathe regularly and sleep soundly.
When we sleep, the airway muscles and tongue relax. For people with OSA, the tongue relaxes into a position that blocks the airway.4 When the airways are blocked, less oxygen is sent to the brain, triggering us to wake up and take a breath. This cycle happens repeatedly, waking up and preventing those with OSA from getting a quality night’s sleep.4
The Inspire implant delivers gentle pulses that move the patient’s tongue out of the way each time they take a breath, keeping the airway open so they can sleep soundly.4 It eliminates the masks and bulky hoses required for CPAP devices. The Inspire device is surgically implanted near the collarbone in a 90-minute outpatient procedure. Most patients require no more than over-the-counter pain medication, and within a few days they can resume their normal daily activities.4
The Inspire device is controlled by the patient through a handheld remote that turns on the implant when they are ready to sleep. The inspire app tracks patients’ sleep goals and allows them to see their sleep data and share it with their health-care providers.
Results of a study
A pivotal study of 126 patients using a hypoglossal nerve stimulation was published in the New England Journal of Medicine in 2014. This nerve stimulation was associated with a 68% reduction in apnea-hypopnea index (AHI), from 29.3 events per hour to 9.0 events per hour in 12 months.5 Sixty percent of subjects achieved a reduction of at least 50% and an AHI of fewer than 20 events per hour.5 The AHI reduction was accompanied by improvements in daytime sleepiness and functional outcomes of sleep as well.5
Qualifying for the Inspire device
Patients who are 18 years of age, have been diagnosed with moderate to severe OSA, and have tried and struggled with CPAP may qualify for Inspire. If patients are interested, they can take a short quiz on the Inspire website. Inspire therapy is even FDA approved for use in pediatric patients with Down syndrome.4
Some contraindications include certain types of sleep apnea disorders, anatomical findings that compromise the performance of airway stimulations, patients who are unable to operate the sleep remote, and those who are pregnant or may become pregnant. Some Inspire models affect MRIs, so patients should consult with their doctor if imaging is necessary.
The company’s website states that most US insurance providers—including Medicare, Veterans Affairs, and major commercial carriers—cover Inspire therapy for those who have tried and struggled with CPAP.4 Most insurance policies have a BMI policy in place for coverage of Inspire therapy. Therapy has been clinically tested for people with a body mass index up to 40.4 Out-of-pocket fees are not listed anywhere on the website.
What dental professionals need to know
So, what do we need to know as oral health-care professionals? Compliance with CPAP is difficult for most patients. One of the most common complaints and reasons for nonadherence to CPAP therapy is dry mouth.6 As oral health-care professionals, we know dry mouth is not only uncomfortable but can put patients at risk for dental caries and other oral infections. This alternative method to treat sleep apnea could be the solution to a better night’s rest, a decrease in systemic health problems, and better oral health due to reduced salivary dryness.
If your patient suffers from sleep apnea and has not had success with traditional CPAP, consider educating them about Inspire. When researching these devices, I spoke with a representative from Inspire and asked if there were instructions and/or contraindications for dental professionals. They said there is no need for any type of premedication with this device, and it will not interfere will ultrasonic use as long as the device remains off during treatment. The company recommends that any wired device should be draped across the patient’s chest opposite of where the implant is placed.
I hope you have found this information to be beneficial. If nothing else, you won’t be caught off guard by those strange wires the next time you take a panoramic on a patient who has the Inspire device.
Disclosures: The author has no relationships with Inspire and has not been paid to write about this product.
Editor's note: This article appeared in the October 2024 print edition of RDH magazine. Dental hygienists in North America are eligible for a complimentary print subscription. Sign up here.
References
- Ling V. Sleep apnea statistics and facts you should know. National Council on Aging. May 8, 2024. Accessed May 14, 2024. https://www.ncoa.org/adviser/sleep/sleep-apnea-statistics/
- Mohammadieh A, Sutherland K, Cistulli PA. Sleep disordered breathing management update. Intern Med J. 2017;47(11):1241-1247. doi:10.1111/imj.13606
- Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
- How Inspire therapy works. Inspire. Accessed May 21, 2024. https://www.inspiresleep.com/en-us/how-inspire-therapy-works/
- The emerging option of upper airway stimulation therapy. Mayo Clinic. February 10, 2018. Accessed May 23, 2024. https://www.mayoclinic.org/medical-professionals/pulmonary-medicine/news/the-emerging-option-of-upper-airway-stimulation-therapy/mac-20431242
- Bortolotti M. The cause of dry mouth during CPAP application. J Clin Sleep Med. 2017;13(4):647. doi:10.5664/jcsm.6568
About the Author

Abbey Rieck, MSEd, BS, RDH
Abbey Rieck, MSEd, BS, RDH, has been a dental hygienist for 14 years in Indianapolis, Indiana. She previously worked in a general family dental practice and as part-time adjunct clinical faculty at the Indiana University School of Dentistry. She is currently a clinical assistant professor at the Indiana University School of Dentistry Dental Hygiene Program. She teaches preventive dentistry and dental health education, and she is the clinical course director for the second-year dental hygiene students.