The need for objective, evidence-based efficacy testing of oral rinses
Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia from the red complex gang bellowed to the bartender, “Pour us some crevicular fluid.”
“Not so fast,” countered Prevotella and Fusobacterium from the archrival orange complex gang. “We’re doing real antiseptic rinse shots here, and you can bet your biofilm that we’ll be the last bugs standing.” And so, the testing began.
Our “periodontal bacteria in a bar story” is a light-hearted introduction to a serious subject—the need for objective, evidence-based efficacy testing of oral rinses against periodontal bacteria in the presence of saliva. Because at-home oral hygiene care is so important in maintaining periodontal health, and oral rinsing is a key component of that critical care, we need the most current and relevant data to support our treatment and product recommendations.
You might also want to read: 8 challenges rinses will resolve in your practice
Although many rinse manufacturers want us to believe otherwise, claims of antiseptic rinse efficacy based solely on laboratory testing in the absence of fresh, human, whole saliva are not sufficiently valid. The salivary proteins in fresh, human, whole saliva effectively neutralize most oral rinses, rendering them ineffective or poorly effective.1 As we will see, when efficacy testing of oral rinses is conducted in the presence of fresh, human, whole saliva, there can be “many, many bugs left standing.” Here's a breakdown on which oral rinses are effective in saliva, which would truly benefit your patients, and which would not.
The efficacy testing results
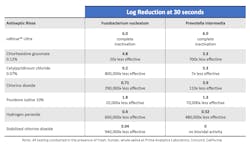
Biocidal efficacy testing of six frequently used professional rinses was conducted at Prime Analytical Laboratories in Concord, California. A newly developed proprietary rinse, ioRinse Ultra (ioTech International, Boca Raton, Florida), was also included to evaluate the efficacy of its unique, patented formulation. Table 1 describes the results of that testing.
The efficacy of each tested rinse in Table 1 is reported by its log reduction of the targeted bacteria—a 1 log reduction = a 10 times reduction, a 2 log reduction = a 100 times reduction, a 3 log reduction = a 1,000 times reduction, and so on.
So, in comparing different log reductions, even a small change can represent an enormous difference in effectiveness. For example, ioRinse Ultra completely inactivated Prevotella intermedia to below detectable limits with a 6.0 log reduction. Chlorhexidine gluconate obtained a 3.3 log reduction of the same microbe under identical test conditions. It doesn’t seem like much of a difference, a 6.0 log reduction versus a 3.3 log reduction, as the difference is only 2.7 logs. But that 2.7 log reduction difference represents a 700 times difference in biocidal efficacy.
The test rinses are shown in descending order of effectiveness. ioRinse Ultra, in attaining 6 log reductions of each of the tested bacteria, was completely successful in reducing those bacteria to below detectable limits. All the other rinses were significantly less effective. Table 1 also shows how much less effective each of the other rinses were compared to ioRinse Ultra.
Also notice in the table that some of the more popular antiseptic rinses tested had little to no biocidal efficacy. All the reported testing occurred in the presence of fresh, human, whole saliva. Salivary proteins can and do neutralize many oral rinses. So, if biocidal efficacy test results are to be useful indicators of actual intraoral biocidal activity, the testing should be conducted in the presence of fresh, whole, human saliva.2
Because ioRinse Ultra was the only rinse found to be fully effective in the testing described in Table 1, it was subjected to additional, independent testing at Nelson Laboratories in Bozeman, Montana, to further validate its efficacy. The testing was conducted at 30-second exposure time in the presence of fresh, human, whole saliva. The two bacteria tested were different than those tested and shown in Table 1 but were also periodontopathic (Porphyromonas gingivalis and Tannerella forsythia). In this further testing, ioRinse Ultra was successful in reducing both bacteria to below the limits of detection within 30 seconds in the presence of saliva.
Daily, at-home use of an antimicrobial mouthrinse by patients has become increasingly important in helping to control periodontal disease. Many key opinion leaders consider it a critical component of comprehensive periodontal care. For example, in a landmark article published in JADA titled “Antimicrobial mouth rinse and the management of periodontal diseases,” the author, Professor Ira Lamster (previous Dean of Columbia University Dental School), emphasized the critical role played by daily at-home rinsing with an antiseptic rinse in managing periodontal disease.3
The director of the ADA Seal of Acceptance program points out that, “Antimicrobial mouthwashes have been shown in clinical studies to prevent the gum disease, gingivitis.”4 Indeed, two randomized, controlled, evaluator-blinded, six-month studies of more than 600 patients showed that twice daily antiseptic rinsing was 34% more effective than daily flossing in reducing interproximal gingivitis, and over 10 times more effective in reducing interproximal plaque.5 A separate, randomized, controlled study of 160 patients conducted at four dental schools over six months concluded that twice daily rinsing resulted in significantly higher anti-plaque and anti-gingivitis effects compared to daily flossing.6 We routinely advocate for patient daily flossing. The evidence suggests that daily, at-home rinsing may be even more important.
Chlorhexidine gluconate performed better than several of the other tested rinses, but since it is indicated for short-term use only, it is not a candidate for long-term, at-home rinsing.7
We know that daily at-home care is as important or more important than in-office treatment because inflammation occurs daily. We have a responsibility to educate and advise our patients. Advising them to use a daily, at-home antiseptic rinse to help control gum disease is critical to maintaining periodontal health. We know that saliva neutralizes many antiseptic rinses, preventing them from working well. We now know which rinses work well in the presence of saliva and can be expected to work well in the mouth.
Editor's note: This article appeared in the May 2023 print edition of RDH magazine. Dental hygienists in North America are eligible for a complimentary print subscription. Sign up here.
References
- ioRince RTU: an effective pre-treatment rinse. Clinicians Report. April 2021;14(3):1-3
- Christensen R. TRAC Research. Personal communication. April 2021.
- Lamster IB. Antimicrobial mouth rinses and the management of periodontal diseases. J Amer Dent Assn. 2006;137(11):55-59.
- Childs D. ADA moves to increase mouthwash awareness. ABC News. May 28, 2007. https://abcnews.go.com/Health/Dental/story?id=3214322&page=1
- Studies show Listerine at least as good as flossing. March 5, 2002. https://www.dentistryiq.com/dental-hygiene/student-hygiene/article/16355416/studies-show-listerine-at-least-as-good-as-flossing
- Mythri H, Ananda SR, Prashant GM, Subba Reddy VV. The efficacy of antiseptic mouth rinse in comparison with dental floss in controlling interproximal gingivitis. J Int Soc Prevent Community Dent. 2011;1(1):31-35. doi:4103/2231-0762.86385
- Goodfellow K. Chlorhexidine–The good, the bad, and the ugly. RDH. August 7, 2022. https://www.rdhmag.com/patient-care/rinses-pastes/article/14173423/chlorhexidinethe-good-the-bad-and-the-ugly
About the Author

Trish Keena, BS, RDH
Trish Keena, BS, RDH, has been an RDH for 15 years and has spent much of her career, in her various roles, researching and advocating for products that make the most impact for patients. Trish has a background in nonprofit work, dental hygiene academia, pharmaceutical sales, and most recently, she serves as the vice president of soft tissue management and clinical support at Elite Dental Partners.