Risks and biohazards associated with dental instrument retipping: A review
In 2013, I completed an original research study that highlighted significant concerns associated with retipping dental instruments, which are considered class 1 Medical Devices requiring clearance by the FDA prior to their sale and distribution.1 This study was borne from discovering retipping as a recently licensed dental hygienist who heard about it at a large regional dental conference. Upon returning home from the conference, I began reading more about the retipping process, and reached out to dental professionals who had conducted previous research. I read and considered the relevant risks to the clinician and patient that were reported by my colleagues and really wanted to see the truth myself.
In late 2012, I embarked on a deep investigation and in-office research study where I obtained retipped instruments from colleagues and compared them to their factory-new counterparts. I did not inquire about where or when the instruments had been retipped, only that each device was clearly marked retipped or refurbished on the handle as required by law.2
Over the course of several months, I conducted a thorough analysis, comparing the cutting edges, angles, balance, and other tactile qualities of new and retipped dental instruments. I gathered 100 retipped instruments from colleagues across the country (and sent them a new instrument in return).
In 2024, over a decade later, these findings remain significant as dental professionals seek cost-effective solutions, in both privately owned practices and larger dental service organizations. While the manufacturing and design of instruments has advanced in this time, the debate about the safety and efficacy of retipped instruments continues. These discussions raise relevant questions including whether retipping impacts the overall instrument integrity, if retipped instruments produce equal effectiveness when it comes to patient outcomes, and if there are additional risks and biohazards for consumers who choose to use retipped instruments chairside.
This review revisits the previous research and discusses additional safety considerations and alternative practices to retipping, as clinicians are reminded of the stringent manufacturing recommendations and standards manufacturers follow when producing quality dental hand instruments.
Trust in manufacturing standards
Manufacturers invest significant time, talent, and money in researching, developing, and maintaining the quality of each instrument that leaves their facilities. Precise care is taken in choosing metals, fabricating each portion of the instrument, sharpening the tips of each specific design, and packaging the products safely for delivery to the practice. Retipping introduces variables that are outside the original manufacturer's control.
When a dental professional purchases a new curette, scaler, probe, explorer, or file, they know they are acquiring a quality piece of hand instrumentation that has been meticulously engineered as a class 1 medical device.3 They know each of these instruments can be relied upon to perform specific tasks both above and below the gumline.
Dental professionals entrust the manufacturers with their own safety and their patients' safety. Patients place trust in their providers that they will receive complete, personalized, and quality dental care. For this reason, manufacturers carefully consider a range of factors, including quality control, material property quality, maintaining a balanced design, ergonomic considerations, and structural integrity of the product over its lifetime.
While these instruments may seem to have similar purposes, dental professionals fully understand that each device is individually and intricately crafted—the shape of the blade, the length of the terminal shank, the depth of the cutting edge, and the weight of the overall product. All these aspects are specifically calibrated and checked for quality.
Each dental instrument manufacturer is known for the details that set their hand instruments apart, yet all companies are committed to maintaining standards set for class 1 medical devices by the Food and Drug Administration (FDA).3 The International Association for Standardization (ISO) also sets standards for medical and dental health-care devices, governing strict quality controls.5 These principles regulate aspects such as consistent material quality, size metrics, the ability to be consistent in shape and maintain that shape throughout the useful life of the device, and overall balance and sharpness.
The importance of metals
Dental instruments today are made primarily of surgical grade stainless steel, with additional elements such as chromium, carbon, nickel, and molybdenum.5 Many manufacturers use proprietary formulas based on the core material 440A, a stainless-steel mixed metal that includes chromium for its resistance to temperature changes during manufacturing and its improvement of the instrument's lifetime performance. This specific grade of metal offers high carbon-steel wear resistance balanced with stainless-steel corrosion resistance, allowing it to withstand sterilization cycles.6
Although manufacturers hold various proprietary recipes for metals they use, all instruments sold within the United States must have clearance from the FDA3 and maintain standards for ISO safety and performance, as proven by routine inspections.4 When federal and international standards are not followed, the instrument integrity can be compromised.
Why retipping is problematic
Instrument manufacturers often hold patents on innovations that have taken place over time. Any alterations in size, balance, quality, weight, or shape result in a new type of instrument—one that needs to be tested for quality and effectiveness prior to distribution. Clinicians are advised against bending or reshaping their own instruments. Processes that alter the cutting edge, bend, or balance of a dental instrument not only may void the existing warranty but also may change the instrument to a degree that it no longer holds the FDA clearance allowing it to be used in patient care.3
How retipping works
Before hearing about retipping in 2012, conducting the research, and publishng the original article in 2013, I was unaware of dental instrument retipping. My accredited dental hygiene school did not discuss the process, and I had not encountered it in my years of private practice. I had limited information as to why dental professionals chose the retipping option, other than cost savings and environmental sustainability.
The 2013 research noted that retipping a dental instrument involves a multistep process that can vary based on the materials of both the tip and handle. Generally, the first step involves the original instrument being sent by a dental practice to the retipper. Then, the retipper forcibly removes the existing instrument tip from the handle, breaking any chemical or physical bonds placed by the original manufacturer. This process may result in microcracks of the handle, and stripping of some of the resin or metal within the handle of the device. Following the removal, a new or refurbished tip is placed in the open handle void, either glued or heat-fit back into the device handle.
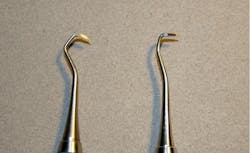
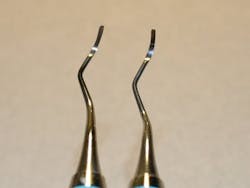
During the process of tip removal and reinsertion, it is very difficult to maintain the original design specifications of the instrument. While the instrument may appear similar after it is retipped, its balance, alignment, and cutting edge can vary greatly. This can be seen in Figures 1 and 2, photos taken during my inspection of retipped instruments. In the original 2013 retipping study, some variations required a micrometer to measure the change in thousandths, while others could be measured with a periodontal probe in millimeters.1
Additionally, the inconsistencies between the handle and the tip in the retipped instruments showed various points of failure. Figure 3 shows the cracks and gaps that can exist following the removal and reinsertion of an instrument tip. Figure 4 also shows that the force applied at the insertion point of the handle can affect the overall integrity of the handle at other wear points.
In the 2013 study, there were various accumulations of debris, corrosion, and fluids trapped in some of the retipped handles. These phenomena likely resulted from submersion of each instrument in ultrasonic cleansing baths, or moisture that was allowed to enter the handle at some point during use but could not then be removed after sterilization processes were completed. This moisture, along with the interaction of dissimilar metals, can contribute to the increase in corrosion incidents for the remainder of the life of the instrument, as seen in Figure 5.
Effects on angulation and ergonomics
During the retipping process, the alterations of both shank and blade angulation may affect the clinician’s ability to consistently perform nonsurgical periodontal care. Even the smallest deviation from the original angulation can contribute to improper working strokes both supra- and subgingivally. In addition, clinicians may have to alter their techniques in biofilm retention area removal (i.e., stain removal or calculus scaling) to prevent damage to the surrounding tissues due to an improperly angled terminal shank of blade. This may also increase the incidence of instrument breakage when force is added to an improperly angled cutting edge or weakened blade, as shown in Figures 1, 6, and 7.
Safety risks of retipping
The Centers for Disease Control and Prevention (CDC) states that breaches in the sterilization process may occur if clinicians do not follow the manufacturer’s instructions for use (IFU).7 Variations between a retipped instrument and a new, FDA-approved instrument that was originally manufactured in a certified facility may increase the risk of bloodborne pathogen transmission to both clinicians and patients. Because a retipped instrument can differ significantly from the original device, the standard IFU may no longer be identical to the original product.
This contributes to one of the most concerning aspects of retipping—the potential impact the process may have on office instrumentation sterilization protocol. The structural alterations made during the retipping process—such as cracks, weakened chemical or physical bonding agents, and gaps in fit—all provide imperfect spaces that may harbor biofilm and biological debris such as blood, cleaning solution, or other infectious agents. These agents can be carried through the sterilization cycle, trapped inside the device. In these instances, even if all the IFU were followed, sterilization procedures may fail.
In addition to sterilization concerns, the corrosion risk from an incompatibility of dissimilar metals or rough junctures between metals may decrease the strength of the device. The cutting edges themselves also showed decreased strength in the 2013 study. This is shown in Figure 1. In another anecdotal report in the 2013 study, a clinician explained that after having several instruments refurbished, the tips started falling out after the first series of sterilization procedures. She was told there was an “adhesive failure” and the instruments would need to be returned for a second series of retipping. The safety risks associated with this specific incidence could have resulted in a more serious patient litigious claim.
Alternatives to retipping
Dental professionals continue to seek value in a tight economic situation. It is critical that budget cuts not create a health risk cost that might be larger than the initial cost savings.
If cost savings are not the primary motivation for retipping, the decision is likely driven by a commitment to environmental sustainability. What if there was more efficient approach to replacing the entire instrument that could ensure each replacement tip and reusable handle provided consistent balance, design, and functionality?
Some companies do offer threaded replacement instrument tips that are custom-built for that exact intention.8 One example is the Quik-Tip system from American Eagle (Young Innovations), an interchangeable system of threaded instrument tips that can be screwed into the end of a standard instrument handle. These types of systems provide both cost savings and a reduction of materials used in manufacturing. In the case of the Quik-Tip system, each end of the instrument can be replaced once it has finished its usable life, without replacing both ends at once. This also allows for customizable instruments for clinicians who want their instruments configured outside what is usually manufactured.
Instrument recycling programs also exist to address the concept of recycling and environmental stability. These programs can be accessed through the various independent instrument manufacturers.
Note that once an instrument is retipped, and marked with the retipped or refurbished engraving, all warranty and customer service accessibility from the original manufacturer is voided. These instruments may no longer be eligible for instrument recycling programs or replacement due to normal handling, as noted in the manufacturer’s instructions for use.
Conclusion
Precision, compliance, and quality are the hallmarks of dental instrument manufacturers who have taken great pride in their work for generations. While dental instrument retipping may offer a lower-cost alternative upfront, the potential risks to patient and clinician safety must be considered.
Compromises in sterilization, instrument breakage, and ergonomics represent significant risk factors. Given these considerations, it is essential to question whether the modest cost savings is justified.
As class 1 FDA medical devices, dental instruments must adhere to standards when leaving the initial manufacturing facility. Safety and effectiveness are both part of the cost of purchasing a product new from the manufacturer. While research indicates that after only 15 strokes, a stainless-steel instrument begins to dull and the bevel begins to change,9 we know that options exist for sharpening stainless-steel or choosing a sharpen-free metal tip option.
After a certain point sharpening is no longer an option, and a decision needs to be made regarding the instrument that has lived its full useful life. Ultimately, this decision rests on the dental professional who is using the device. It is my intent to explain the concerns raised from the initial 2013 study and provide additional details to consider in this dive into the current regulation and options for replacing worn devices. Maintaining the highest standards of dental practice and patient safety remain top priority, and it is always our professional ethical responsibility to place reliability and efficacy over cost.
Editor's note: The article appeared in the November/December 2024 print edition of RDH magazine. Dental hygienists in North America are eligible for a complimentary print subscription. Sign up here.
References
- Boge E. Scary, nasty, and shocking. RDH magazine. November 12, 2013. https://www.rdhmag.com/patient-care/article/16406305/scary-nasty-and-shocking
- Remanufactured devices: Ensuring their safety and effectiveness. Medical Devices and Diagnostic Industry. January 1, 1997. https://www.mddionline.com/manufacturing/remanufactured-devices-ensuring-their-safety-and-effectiveness
- Quality system regulation/medical device current good manufacturing practices. Food and Drug Administration. 2024. Accessed October 21, 2024. https://www.fda.gov/medical-devices/quality-system-qs-regulationmedical-device-current-good-manufacturing-practices-cgmp/medical-devices-current-good-manufacturing-practice-cgmp-final-rule-quality-system-regulation
- 1988 ISO/TC 106/SC 4 Dental instruments. International Organization for Standardization. 2024. https://www.iso.org/committee/51296.html
- Go HB, Bang JY, Kim KN, Kim KM, Kwon JS. Mechanical properties and wear resistance of commercial stainless steel used in dental instruments. Materials (Basel). 2021;14(4):827. doi:10.3390/ma14040827
- Stainless steel type 440A (UNS S44002). TMS Tempered and Specialty Metals. 2024. https://temperedmetals.com/stainless-steel-type-440a/
- Centers for Disease Control and Prevention. MMWR guidelines for infection control in dental healthcare settings. MMWR Recomm Rep. 2003;52(RR-17). https://www.cdc.gov/mmwr/PDF/rr/rr5217.pdf.
- Quik-Tip interchangeable instrument tips. American Eagle. 2024. Accessed October 21, 2024. https://www.am-eagle.de/en/quik-tip-exchangeable-dental-instrument-tips/
- Tal H, Panno JM, Vaidyanathan TK. Scanning electron microscope evaluation of wear of dental curettes during standardized root planing. J Periodontol. 1985;56(9):532-536. doi:10.1902/jop.1985.56.9.532
About the Author

Emily Boge, EdD, RDH, CDA, FAADH, FADHA, CDIPC
Wife, mother, farmer, college administrator, educator, inventor, public health advocate, businesswoman, researcher, writer, editor, speaker—yet always a dental hygienist—Emily Boge, EdD, RDH, CDA, FAADH, FADHA, CDIPC, has worn many hats over 20-plus years in the dental industry. She takes pride in utilizing her inquisitive mind and honest attitude to lead faculty at her college, influence manufacturers to listen to dental professionals in product innovation, and transform students into entry-level professionals, promoting the use of inner accountability, tenacity, and empowerment. Dr. Emily currently serves as dental administrative chair at Hawkeye Community College in Waterloo, Iowa, and owns a farm and the speaking, consulting, and writing firm Think Big Dental.