Untreated periodontitis and COVID-19? Let’s get progressive! Part 3 of 3
Editor's note: In part 1 and part 2 of this series, published in October and November, respectively, I proposed that untreated periodontitis could compromise immunity or contribute to complications of pandemic-scale viruses such as SARS-CoV-2 and offered two biological mechanisms to explain this potential association.
Treating periodontitis has always been important, but during this pandemic I suggest that we need to become more strategic than ever in caring for our patients, especially those who are at risk for periodontitis. In this third and final part of the series, I suggest four aspects of progressive periodontal therapeutics that could make a difference in the periodontal stability of our patients:
- Reigniting compliance to self-care instructions
- Updating our patient education with the most current information about how untreated periodontitis may influence susceptibility to viral illness
- Improving the accuracy of probing to measure changes that determine periodontal stability
- Incorporating adjunctive therapies that help stabilize the reduced periodontum
Encouraging patients to comply with self-care instructions
Today we know that only 20% of the risk of developing periodontitis is plaque related; the lion’s share of risk is actually from risk factors unrelated to plaque.1 Nonetheless, good oral hygiene has always been thought of as the cornerstone of periodontal health,2 and today, the reduction of bacterial burden anywhere in the body is important. In their contribution to the 2017 system for Classification of Periodontal and Peri‐Implant Diseases and Conditions, Lang and Bartold suggested that “. . . for periodontal health to be attained, or maintained, the composition of the subgingival microbiota needs to be redirected toward one compatible with gingival health.”2 So how do we motivate our patients to better comply with recommendations for self-care?
Over the last 30 years, various models have attempted to explain the factors in achieving greater compliance to oral hygiene instructions in adult periodontal patients. In 2015, a systematic review of 15 articles related to these models concluded that a key predictor of compliance is when patients understand the seriousness of periodontal disease and the benefit of improving oral hygiene habits.3 Consequently, the responsibility to educate patients that untreated or unstable periodontitis may pose a threat to overall health falls to oral health-care providers (OHCPs). This may be even more important during a viral pandemic. But, a note of caution here: what we say must be grounded in evidence. Evidence-based sound bites for patient education on this subject will follow.
It’s also important to make it easy for patients to comply with our recommendations for self-care. Offering alternatives to specific self-care procedures boosts compliance. As an example, insisting that a patient use floss may sabotage his/her best attempts to improve oral hygiene. Flossing is a gold standard. Yet, for a number of reasons, there are many people who simply can’t or won’t floss. In this case, you can suggest the use of other interdental cleaning devices such as picks as a great alternative to flossing.
Updating our evidence-based patient education
We should provide evidence-based patient education that zeros in on the possibility that untreated periodontitis could influence susceptibility to viruses and/or escalate the severity of related complications of viral illnesses. What can we say to our patients that is grounded in evidence? Here are sound bites, backed by science, that are not meant to be alarmist but rather help patients see the bigger picture in terms of health risk. These messages are not intended to scare patients into treatment, but for those who have questioned the benefit of periodontal therapy, this information may be beneficial.
- One of the strongest defenses we have against viral infections such as COVID-19 is a robust immune system so we can fight infection. That’s why we’re doing our best to help our patients boost their immunity.
- There are a number of scientifically based recommendations to help boost your immune system, such as eating nutritious foods and balancing your diet, making sure to get adequate sleep and physical activity, getting outdoors for some sunshine, and managing stress.
- But also, research suggests that treating chronic infections, including in the mouth, may help improve immunity.
- Poor oral health influences immunity, and if periodontal disease (gum disease) is left untreated, it may negatively impact your overall immunity.
- For many years we’ve known that bacteria from gum disease can be aspirated into the lungs. When this happens, the lungs’ ability to fight infection is disrupted, which may increase the risk for viral coinfections, such as viruses like COVID-19.
- That’s why, more than ever before, we’re being especially diligent in examining patients for gum disease or any signs of oral inflammation. And, for those who have gum disease, it’s important to get treated as soon as possible.
- Thorough oral hygiene will reduce the bad bacteria in your mouth. This includes brushing your teeth twice a day with an antimicrobial toothpaste and cleaning in-between your teeth with floss or picks. Water irrigation devices and antimicrobial mouthwashes are other tools for keeping your mouth clean. The goal is to decrease the bacterial burden in your mouth.
Measure periodontal metrics that determine stability
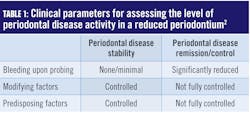
Improving the accuracy of probing and measuring changes that help determine periodontal stability by utilizing an automated probing system are vital to progressive periodontal therapies. Preserving periodontal health over a lifetime, albeit theoretically possible, is unrealistic for most people. Because in periodontitis there are periods of dormancy and bursts of destruction, we know the progression of the disease is not a straight line. Fortunately, the 2017 classification system addressed this by suggesting a new therapeutic target for patients with a reduced periodontium (after initial treatment).2 Low disease activity has been specified as an acceptable alternative therapeutic goal, particularly in long‐standing disease. Clinical parameters that inform the level of disease activity can be seen in Table 1.For patients with a reduced periodontium, the 2017 classification system defined two types of treatment outcomes: stability and remission/control. In a periodontium reduced by previous disease activity, the goal must be to achieve stability or remission/control. The status or stability of a case is assessed by what has been termed “guiding stars” in formulating treatment plans and determining the best strategies and intervals for periodontal maintenance.
Periodontal disease stability is defined as a state in which the disease has been successfully treated and clinical signs do not appear to worsen in extent or severity despite the presence of a reduced periodontium. Periodontal disease remission/control is defined as a period in the course of disease when symptoms become less severe but may not be fully resolved.2
Modifying factors are dynamic changes that influence the level of disease activity. A good example is an HbA1ci of greater than 7.0%, which would increase the risk for periodontal disease activity. Predisposing factors are factors that increase susceptibility to periodontal disease activity. Smoking, a known risk factor for periodontal disease, would be an example of a predisposing factor.
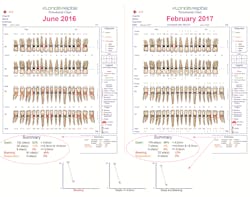
An automated probing system, such as the Florida Probeii technology, is an essential tool in operationalizing the new 2017 diagnostic framework. The Florida Probe handpiece was developed at the University of Florida in 1985 in response to a request by the National Institutes of Health and received FDA approval in 1987. Beyond its strong track record for greater probing accuracy, efficiency in the operatory, beautiful printout of exam results, and increased acceptance of periodontal treatment plans, Florida Probe has another feature that is pivotal in informing our clinical decisions. The system tracks metrics that are key in determining staging, grading, and the level of disease activity in a reduced periodontium (figure 1).These Florida Probe charts provide a comparison of clinical metrics before treatment and five months after treatment. The callouts demonstrate how the Florida Probe technology automatically calculates a summary of the key metrics, such as the number of sites with pockets less than 4 mm, sites with pockets between 4 and 5 mm in depth, and sites with pockets greater than 5 mm, in addition to the number of sites that bleed upon probing (BOP) and sites of suppuration. The line graphs on the bottom indicate the changes that occur, such as pocket depths and BOP, at subsequent examinations. This information assists clinicians in determining staging and grading in the diagnosis of periodontitis, and the level of disease activity in a reduced periodontium that has been defined by the 2017 Classification of Periodontal & Peri-Implant Diseases and Conditions.
Incorporate into periodontal maintenance protocols adjunctive therapies that facilitate periodontal disease stability in patients with a reduced periodontium
There are two strategies that clinicians should consider adopting in caring for patients after initial periodontal treatment.
- Host-modulatory therapy with subdose doxycycline reduces degradation of the collagen within the periodontium.
- A custom-fabricated tray system that utilizes hydrogen peroxide targets pathological bacteria and creates ecological conditions that make survival of periodontal pathogens unsustainable.
The use of subdose doxycycline
Pharmacological modulation of the host response (host-modulatory therapy) is a modality that can be used to help preserve the collagen content of the periodontium. Over two decades ago, Golub and colleagues discovered that tetracycline could inhibit connective tissue breakdown,4 and subsequent studies affirmed that the use of a subantimicrobial dose of doxycycline as an adjunct to scaling and root planing (SRP) improved clinical parameters beyond what can be achieved by SRP alone.5,6
Doxycycline at a dose as low as 20 mg twice a day does not reach the minimum inhibitory concentration necessary to cause an antimicrobial effect; therefore, it can be used long term in helping to stabilize the reduced periodontium. Subdose doxycycline as an adjunct for periodontal therapy was originally produced under the label Periostat,iii and it is still marketed under this name. Today, generic doxycycline, at 20 mg twice a day, can also be prescribed. To my knowledge, this adjunctive therapy, though proven to be successful, has not been widely adopted, and that is unfortunate. Its robust evidence base suggests it is a valuable adjunctive therapy to SRP, and I encourage clinicians to consider using Periostat or its generic equivalent.
The use of a custom-fabricated tray system with hydrogen peroxide
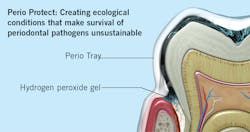
One of the most powerful tools we have to preserve the homeostasis of periodontal tissues achieved following SRP is a tray delivery system that disperses and sustains optimal levels of hydrogen peroxide in periodontal pockets as deep as 9 mm. Perio Trays,iv cleared by the FDA, are designed with a patented internal peripheral seal and extension system that works like a gasket to prevent hydrogen peroxide from leaking into the mouth. The tray system is customized for each patient so that the medication can be delivered into patient-specific sites. The system uses hydrogen peroxide, at a concentration of 1.7%, called Perio Gel. With concern about bacterial resistance from overprescribing of antibiotics, Perio Gel’s lack of bacterial resistance is another reason to consider this adjunct in stabilizing the reduced periodontium.
As the hydrogen peroxide activates, it lyses the cell walls of pathogenic bacteria, killing the cells and oxygenating subgingival tissues. This process has the capacity to modify the environment of the subgingival microbiota in a way that survival of periodontal pathogens becomes unsustainable (figure 2).Conclusion
Could untreated periodontitis contribute to increased risk for COVID-19 and/or the severity of its complications? Like many other responses from scientists who lead us during this pandemic, we just don’t know yet. However, what we do know is that treatment of periodontitis can lower overall systemic inflammation. Let’s start there.
More viral influenzas are coming, so what can we do to prepare for these future assaults? Could treatment for chronic infections such as periodontitis be a preemptive strategy to mitigate the risk of infection posed by future viruses such as SARS-CoV-2? The view that periodontitis is an infection limited to the oral cavity without systemic consequences prevents us from assuming the responsibility (and accountability) we must take for the overall health of our patients. Many years ago, I wrote, “The science indicates that as individual clinicians, we have the potential to profoundly change the course of serious inflammatory diseases.” Never could I have imagined a time when this statement would be even more relevant.
Comments are welcome.
Disclosure: Florida Probe and Perio Protect provided unrestricted educational grants to Casey Hein for her research of this subject and writing of this article.
Notes
- HbA1c, also known as glycated hemoglobin, is produced when glucose in the blood sticks to hemoglobin, a protein within red blood cells required for the transportation of oxygen. HbA1c (blood test) is a snapshot of the average blood sugar levels in a person with diabetes over a period of time, usually three to four months. The more glucose in the blood, the more glycated hemoglobin is produced. High blood glucose levels are associated with increased risk for complications of diabetes. The HbA1c goal for most people with type 2 diabetes is less than 7%.
- Florida Probe Corporation. Gainesville, FL.
- Marketed by Galderma Laboratories, L.P. Fort Worth, TX; manufactured by PMRS, Inc. Horsham, PA.
- Perio Protect LLC, St. Louis, MO.
References
- Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65(3):260-267.
- Lang NP, Bartold PM. Periodontal health. J Periodontol. 2018;89(Suppl 1):S9-S16.
- Newton TJ, Asimakopoulou K. Managing oral hygiene as a risk factor for periodontal disease: a systematic review of psychological approaches to behaviour change for improved plaque control in periodontal management. J Clin Periodontol. 2015;42(Suppl 16):S36-S46.
- Golub LM, Lee HM, Ryan ME, et al. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12(2):12-26.
- Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71(4):521-532.
- Preshaw PM, Hefti AF, Jepsen S, et al. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. J Clin Periodontol. 2004;31(9):697-707.
- Keller DC, Cochrane B. Composition of microorganisms in periodontal pockets. J Oral Health Dent. 2019;2(2):123-36.
- Cochrane RB, Sindelar B. Case series report of 66 refractory maintenance patients evaluating the effectiveness of topical oxidizing agents. J Clin Dent. 2015;26(4):109-114.
- Putt M, Mallatt M, Messmann L, et al. A 6-month clinical investigation of custom tray application of peroxide gel with or without doxycycline as adjuncts to scaling and root planing for treatment of periodontitis. Amer J Dent. 2014;27(5):273-284.
- Putt M, Proskin H. Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: results of a randomized, controlled trial after 6 months. J Clin Dent. 2013;24(3):100-107.
- Putt M, Proskin H. Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: a randomized, controlled three-month clinical trial. J Clin Dent. 2012;23(2):48-56.
Casey Hein, MBA, BSDH, RDH, is an internationally recognized speaker and extensively published author with more than 40 years’ experience as a dental hygienist in private practice, public health, education, and government. She first began speaking about periodontal-systemic links in 2003 and founded the first publication on oral-systemic science, Grand Rounds in Oral-Systemic Medicine. She is a pioneer in implementation of periodontal-systemic science, medical-dental collaboration, and providing primary-care services traditionally delivered by physicians and nurses in dental offices. Contact her at caseyhein.com.