Contemporary periodontal recommendations should be made using current evidence-based science that focuses on treating a human patient—not just their oral cavity. A periodontal paradigm shift occurred in 2018 with the release of the new Classification of Periodontal and Peri-implant Diseases and Conditions that changed the identification, classification, and management of periodontal and peri-implant diseases.1 The classification connects the oral microbiome with a patient’s systemic microbiome.
Oral microbiome
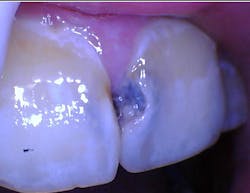
The oral microbiome is a diverse community of microorganisms such as bacteria, fungi, protozoa, viruses, and genetic materials.2-4 A symbiotic environment exists when those microorganisms are in balance and in homeostasis with one another. A dysbiotic environment occurs when the microorganisms are in disharmony with one another and results in disease (figure 1).
The relationship between the host (human) and the oral microbiome is dynamic. A divergence from oral symbiosis into dysbiosis causes profound physiological effects throughout the body. The goal of preventive dentistry is to reestablish, or maintain, a symbiotic oral microbiome to ward off disease.
Biofilm
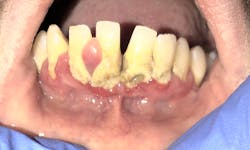
The microorganisms of the oral microbiome do not live alongside one another as independent unicellular beings. They form highly regulated, complex, organized communities that attach to hard and soft tissues in form of an oral biofilm (figure 2).5
The oral biofilm in periodontal health remains stable over time and exists in a state of biologic equilibrium and homeostasis, ensuring no one bacterial strain dominates over another.3 When oral biofilms grow uncontrolled, an imbalance of organisms can occur in which pathogenic (capable of causing disease) bacteria dominate and less pathogenic bacteria are suppressed.3 The new biofilm complex favors dysbiosis in which a host immune response may occur in a susceptible individual who has one or more identified risk factors.6-8
You might also be interested in ...
Do you really know what oral-systemic means?
Retiring the "cleaning lady": A futuristic approach to treating oral disease
Biofilm and its role in periodontal disease
In the past, there were two perspectives on the roles that bacteria and biofilm play in the initiation of periodontal disease: the nonspecific plaque hypothesis and the specific plaque/microbial shift hypothesis.3 These perspectives have recently been debunked and replaced with three new contemporary theories: the ecological plaque hypothesis, microbial homeostasis-host response hypothesis, and keystone pathogen theory. All three are principled under the same recognition that:
- A pathogenic biofilm complex is a prerequisite for disease initiation, but its simple presence does not always lead to attachment loss.3 It is the uncontrolled host immune inflammatory response that causes tissue and bone destruction.3,9
- There is no one bacterial strain that is an initiator of periodontal disease.3
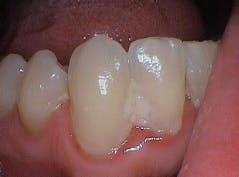
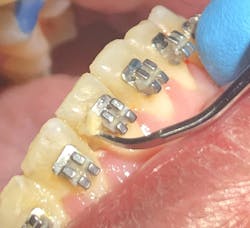
Oral health-care providers are charged with reducing the level of pathogenic microorganisms in an oral biofilm that stops the host immune response and resolves the clinical signs and symptoms of disease (inflammation). Complete pathogenic bacterial removal is not possible or needed to achieve oral symbiosis.3,9 Providers use both mechanical (physical removal; figure 3) and nonmechanical (in-office lasers, locally administered subgingival medicaments, at-home chemical agents) approaches for biofilm removal to achieve these goals.
Disease management
When pathogenic biofilm initiates disease (gingivitis, periodontal disease, peri-implant disease) in a susceptible host, the oral health-care provider must develop a treatment plan framework that includes debridement approaches, personalized care recommendations based on individual risk factors, and long-term monitoring of the patient’s response to therapy. This is the act of practicing periodontal medicine as outlined in the 2018 classification system: considering all individual patient needs, supplemental biologic characteristics, estimation of the rate and likely progression of disease when diagnosing, strategizing, and treatment planning.1
Providers should use the staging and grading framework from the classification system for disease treatment planning and maintenance recommendations:
- Stages I and II: Patients have a good prognosis with easy-to-understand complexity.8,9 Nonsurgical periodontal therapy should be performed as the first line of recommended treatment with the goal of inflammation resolution and a return to oral symbiosis.3
- Stages III and IV: Patients have an increased risk of future tooth or dentition loss.8,9 These patients have an increased complexity of disease management and poorer prognosis. They may require both nonsurgical and surgical interventions.
- Grade A: Patients have slowly progressing periodontal disease and a good prognosis with nonsurgical periodontal therapy and three- to six-month maintenance recalls.3,8,9
- Grades B and C: Patients have moderately (B) and rapidly (C) progressing periodontal disease.8,9 They require more frequent maintenance recalls (every one to three months) and will likely benefit from nonmechanical debridement therapies to control their disease.3,10
Systemic antibiotics
Systemic antibiotics are no longer used as a standard of care in periodontal and peri-implant disease management.3 While planktonic bacterial cells are affected by antibiotics, an oral biofilm provides a shield over the individual bacterial cells with its extracellular polysaccharide matrix.11 The antibiotic dosage needed to penetrate through the shield to reach the individual cells would have to be 1,000–1,500 times its regular dosage, which is enough to kill the patient before it kills the bacteria in the biofilm.3,11 Decades of evidence-based research have shown that periodontal and peri-implant diseases respond favorably to nonsurgical interventions, and antibiotics are seldom needed to gain control of disease pathogenesis.3
Chemical agents
Chemical agents are frequently used as part of a comprehensive treatment plan for patients with active disease. They are typically used as an adjunct to nonsurgical therapies and can be helpful in deep periodontal pockets, unresolved inflammation, and overall biofilm reduction and control.12 According to the American Academy of Periodontology (AAP),12 for a chemical agent to be effective, it must meet three criteria:
- Reach the base of the periodontal pocket
- Have bacteriostatic or bactericidal properties
- Remain in place long enough to exert its action
Controlled clinical trial data and long-term maintenance studies show the benefits of reducing gingival inflammation by delivering a low dose (1.7%) hydrogen peroxide gel (Perio Gel, QNT Anderson) in an FDA-cleared, lab-made, custom-fabricated prescription tray (Perio Tray, Perio Protect LLC) for patient at-home use.13-18 Per the Food and Drug Administration (FDA), Perio Tray is a “custom fit tray requiring an impression from a licensed dentist used to place solutions into gingival crevices or periodontal pockets and permits the placement of solutions deeper into the crevice/pocket than is possible with traditional fluoride trays.”19
Hydrogen peroxide (H2O2) is one of the oldest, cleanest, and most versatile antimicrobial agents on the planet. It is naturally produced by humans as a by-product of the biochemical metabolism of many different cells.20 It is present in the lungs, gastrointestinal system, and bloodstream.20 Even oral bacteria produce hydrogen peroxide that becomes part of the exhaled air by humans.20
H2O2 decomposes to water and oxygen by organisms containing specific enzymes (catalase, peroxidase) that break it down, such as human cells, aerobic bacteria, and facultative anaerobic bacteria.20,21 Obligate anaerobic bacteria do not possess these enzymes, and when exposed to hydrogen peroxide, they have a toxic effect through damage to their cellular lipids, nucleic acids, and cell membranes.20,21 This makes H2O2 a useful chemical agent in the fight against inflammation caused by a host-mediated immune response to oral biofilm.
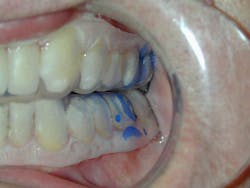
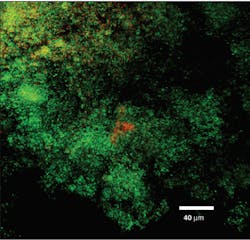
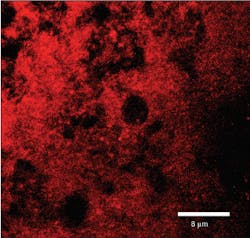
Perio Gel is placed inside a Perio Tray (figure 4) for an average time of 15 minutes per session. Through simple diffusion, the gel will reach the base of the periodontal pocket, sustain at high levels for a long period of time, and exert both bactericidal and bacteriostatic effects (figure 5). Perio Gel alters the microbiological state of the subgingival environment through increasing subgingival oxygen saturation, which makes it harder for obligate anaerobes to thrive. A study published in the Journal of Clinical Dentistry confirmed the bactericidal capability of Perio Gel in 2011.22 Facultative anaerobic Streptococcus mutans was exposed to Perio Gel for 10 minutes, and virtually all cells died as evident by the color change from green (S. mutans, untreated control; figure 6a) to red (dead cells; figure 6b).22 This same study also confirmed that Perio Gel delivered in a Perio Tray was able to diffuse down to 9 mm pocket depths.
The Perio Tray delivery system is a helpful nonmechanical biofilm disruptor for patients with risk factors such as described above. It meets the AAP criteria for chemical agent effectiveness by increasing oxygen concentration both supragingivally and subgingivally. Another advantage is that oral bacteria do not develop resistance to peroxides as they do to antibiotics.
- Oral aerobic bacteria may respond to the increase in H2O2 introduction in the mouth over time and increase their catalase enzyme numbers; however, remember that catalase decomposes H2O2 to oxygen and water in the mouth, which makes it even harder for obligate anaerobes to thrive.
- Obligate anaerobes do not possess catalase. They do produce superoxide dismutase that breaks down the superoxide radical causing the release of peroxide and oxygen.23 Again, increasing the oxygen saturation in the mouth will change the oral microbiome composition by decreasing obligate anaerobic concentrations, which favors symbiosis by creating the right conditions for healthy bacteria to repopulate.
Supplemental testing
There are multiple companies that offer genetic, bacterial, and gingival crevicular fluid (GCF) content testing either through saliva or periodontal pocket cultures. The specific bacteria and genetic markers tested vary by manufacturer. Research has not identified one test as superior to another.3 At this time, supplemental testing should not be routinely ordered for a patient with periodontal disease.3 A stage III or IV and/or grade B or C patient who is unresponsive to initial nonsurgical interventions may benefit from supplemental testing.
Providers should exercise caution in interpreting results when a manufacturer is reporting Socransky’s Microbial Complex grouping of bacterial species as this was used with the historical perspectives of biofilm’s role in periodontal disease. There is no current evidence to support the argument that red and orange complex bacteria are the initiators of disease; rather, it is the uncontrolled host immune inflammatory response that initiates periodontal disease.3,9
Dental providers should also exercise caution if a manufacturer is recommending continued supplemental testing or elimination of a specific pathogen in the absence of active disease. If the oral microbiome is in symbiosis, alterations to the microbial content are not indicated and do not support the practice of contemporary periodontal medicine.
Conclusion
Patients will benefit from seeing providers who use evidence-based science to drive their periodontal treatment recommendations. The goal of periodontal intervention is to reduce the level of pathogenic organisms that stops the host immune response and the clinical signs and symptoms of disease.
Editor's note: This article appeared in the April 2023 print edition of RDH magazine. Dental hygienists in North America are eligible for a complimentary print subscription. Sign up here.
References
- Canton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions – introduction and key changes from the 1999 classification. J Periodontol. 2018;89(Suppl 1):S1-S8. doi:10.1002/JPER.18-0157
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721-5732. doi:10.1128/JCM.43.11.5721-5732.2005
- Gehrig JS, Shin DE, Willmann DE. Foundations of Periodontics for the Dental Hygienist. 5th ed. Wolters Kluwer; 2019.
- Segre J. Microbiome. National Human Genome Research Institute. Accessed December 30, 2022. https://www.genome.gov/genetics-glossary/Microbiome
- Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome – an update for oral healthcare professionals. Br Dent J. 2016;221(10):657-667. doi:10.1038/sj.bdj.2016.865
- Albandar JM, Susin C, Hughes FJ. Manifestation of systemic diseases and conditions that affect the periodontal attachment apparatus: case definitions and diagnostic considerations. J Periodontol. 20158;89(Suppl 1):S183-S203. doi:10.1002/JPER.16-0480
- Lang NP, Bartold PM. Periodontal health. J Periodontol. 2018;89(Suppl 1):S9-S16. doi:10.1002/JPER.16-0517
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159-S172. doi:10.1002/JPER.18-0006
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S173-S182. doi:10.1002/JPER.17-0721
- Darcey J, Ashley M. See you in three months! The rationale for the three monthly periodontal recall interval: a risk based approach. Br Dent J. 2011;211(8):379-385. doi:10.1038/sj.bdj.2011.868
- Park SC, Park Y, Hahm KS. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci. 2011;12(9):5971-5992. doi:10.3390/ijms12095971
- American Academy of Periodontology statement on the local delivery of sustained or controlled release antimicrobials as adjunctive therapy in the treatment of periodontitis. J Periodontol. 2006;77(8):1458. doi:10.1902/jop.2006.068001
- Cochrane RB, Sindelar B. Case series report of 66 refractory maintenance patients evaluating the effectiveness of topical oxidizing agents. J Clin Dent. 2015;26(4):109-114.
- Keller DC, Cochrane B. Composition of microorganisms in periodontal pockets. J Open Humanit Data. 2019;2(2):123-136.
- Keller D, Buechel M, Sawyer G, Goldstein J. Managing peri-implantitis and peri-mucositis with direct medication delivery. Int J Dent & Oral Heal. 2017;3(10):103-116. doi:25141/2471-657X-2017-10.0103
- Putt MS, Mallatt ME, Messmann LL, Proskin HM. A 6-month clinical investigation of custom tray application of peroxide gel with or without doxycycline as adjuncts to scaling and root planing for treatment of periodontitis. Am J Dent. 2014;27(5):273-284.
- Putt MS, Proskin HM. Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: results of a randomized, controlled trial after six months. J Clin Dent. 2013;24(3):100-107.
- Putt MS, Proskin HM. Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: a randomized, controlled three-month clinical trial. J Clin Dent. 2012;23(2):48-56.
- 501(k) summary for Perio Protect Tray. US Food and Drug Administration. Department of Health & Human Services. January 23, 2004. Accessed July 2, 2021. https://www.accessdata.fda.gov/cdrh_docs/pdf3/K031809.pdf
- Mayo L. Hydrogen peroxide: it’s not just for cuts and scrapes anymore…explore its oral applications. Dental Learning. 2022. Accessed December 20, 2022. https://www.dentallearning.net/course/HYPRA-CE
- Boateng MK, Price SL, Huddersman KD, Walsh SE. Antimicrobial activities of hydrogen peroxide and its activation by a novel heterogeneous Fenton’s-like modified PAN catalyst. J Appl Microbiol. 2011;111(6):1533-1543. doi:10.1111/j.1365-2672.2011.05158.x
- Dunlap T, Keller DC, Marshall MV, et al. Subgingival delivery of oral debriding agents: a proof of concept. J Clin Dent. 2011;22(5):149-158.
- Rocha ER, Selby T, Coleman JP, Smith CJ. Oxidative stress response in an anaerobe, Bacteroides fragilis: a role for catalase in protection against hydrogen peroxide. J Bacteriol. 1996;178(23):6895-6903. doi:10.1128/jb.178.23.6895-6903.1996