'Needleless' intranasal spray: Kovanaze product for local anesthesia makes its debut
By Laura Webb, RDH, MS, CDA
A much anticipated "needleless" intranasal spray dental local anesthetic received Federal Drug Administration (FDA) approval on June 29. Kovanaze, containing 3% tetracaine HCl (ester) and 0.05% oxymetazoline HCl (vasoconstrictor) has been in development over the last 10 years by St. Renatus, LLC, based in Fort Collins, CO.
The product is named after the patron saint of anesthesia. The company was founded for the purpose to develop, commercialize, and distribute the first known intranasal dental local anesthetic spray.
Topical tetracaine has been safely and effectively used intranasally by the ear-nose-throat (ENT) medical community for ENT procedures. Patients noticed that their upper teeth felt numb, which led to interest for application of this type of anesthesia for dentistry. The oxymetazoline (the active ingredient in Afrin and other nasal decongestant sprays) was added to Kovanaze to slow systemic absorption of the tetracaine and improve duration of action.1,2,3
The following information is adapted from the product insert: 4
"Kovanaze is indicated to provide regional pulpal anesthesia when performing restorative procedures on teeth #4-13 and A-J for individuals who weigh 40kg or more." It is formulated in a pre-filled, single-use nasal sprayer: 6 mg tetracaine HCl and 0.1 mg oxymetazoline HCl (equivalent to 5.27 mg tetracaine and 0.088 mg oxymetazoline) in each 0.2 mL spray. It is intended for intranasal use only, and administration should be on the same side to the maxillary tooth being treated (see Table 1: Dosage). It is recommended to wait 10 minutes after administration and then determine anesthesia effectiveness. The same sensations of numbness or tingling of soft tissues, commonly felt by patients with injected local anesthesia, may not be experienced.
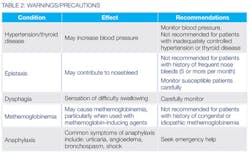
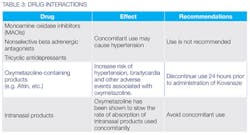
Contraindications include known hypersensitivity to: tetracaine; other ester local anesthetics; p-amino benzo acid (PABA); benzyl alcohol; or any other product in compound. Warnings/Precautions and Drug Interactions are summarized in Tables 2 and 3.
In more than 10% of patients, the most common adverse reactions included rhinorrhea, nasal congestion, increased lacrimation, nasal discomfort, and oropharyngeal pain. Transient, asymptomatic elevations in systolic blood pressure (> 25mm Hg from baseline) and diastolic blood pressures (> 15 mm Hg from baseline) have been reported.
Studies have shown that intranasal tetracaine/oxymetazoline spray is generally safe and well-tolerated with no serious side effects in study participants.2,3,5
At the time this article was written (August 2016), limited data was available regarding the use of Kovanaze for pregnant, pediatric, and geriatric patients. Cianco et al. suggest that this type of anesthesia may be particularly useful for children since children have better circulation and a smaller area for agent delivery.2 Additional safety and efficacy data including the use of Kovanaze for specific populations will become available over time.
Periodontal Therapy
Although the approval focused on restorative procedures, we may find that Kovanaze can also be useful for our hygiene patients, particularly those who require only pulpal anesthesia in the maxillary anterior and premolar regions. The effect on soft tissues associated with scaling and root planning has not yet been determined, although the Ciancio et al. clinical trial, which included testing of the incisive papilla and the palatal foramen, reported successful anesthesia in those areas. The authors suggested that this may be due to the proximity of the nasal spray target to the greater palatine nerve and maxillary sinus.2 The study also indicated some success for pulpal anesthesia for maxillary first permanent molars.1,2
St. Renatus, LLC, continues to investigate the anatomical effects and possibilities for clinical use of Kovanaze. For more detailed information contact St. Renatus, LLC, directly.6 Full prescribing information is available from the product package insert.4RDH
References
1. Malamed S. Handbook of Local Anesthesia. 6th ed. St. Louis, MO: Elsevier; 2013.
2. Ciancio SSG, Hutcheson MC, Ayoub F, Panera Jr EA, Pantera CT, Garlopo DA, Sobieraj BD, Almubarak SA. Safety and efficacy of a novel nasal spray for maxillary dental anesthesia. J DENT RES July 2013 92: S43-S48, first published on May 20, 2013.
3. Hersh EV, Pinto A, Saraghi M, Naleed S, Pulaski L, Gordon SM, Barnes D, Kaplowitz G, bloom I, Sabti M, Moore P, Lee S, Meharry M, He DY, Li Y. Double-masked, randomized, placebo-controlled study to evaluate the efficacy and tolerability of intranasal K305 (3% tetracaine plus 0.05% oxymetazoline) in anesthetizing maxillary teeth. JADA Apr 2016 Vol 147 (4): 278-287.
4. Product Package insert: http://st-renatus.com/wp-content/themes/st-renatus/assets/images/Kovanaze-Prescribing-Information.pdf accessed Aug 2, 2016.
5. Giannakopoulos H, Levin L, Chou J, Cacek A, Hutcheson M, Secreto S, Moore P, Hersh E. The cardiovascular effects and pharmacokinetics of intranasal tetracaine plus oxymetazoline. JADA Aug 2012 Vol 143 (6): 872-880.
6. St Renatus LLC: http://st-renatus.com/ Customer support: (800) 770-9400.
LAURA J. WEBB, RDH, MS, CDA, is an experienced clinician, educator, and speaker who founded LJW Education Services (ljweduserv.com). She provides educational methodology courses and accreditation consulting services for allied dental education programs and CE courses for clinicians. Laura frequently speaks on the topics of local anesthesia and nonsurgical periodontal instrumentation. She was the recipient of the 2012 ADHA Alfred C. Fones Award. Laura can be contacted at [email protected].